Abstract
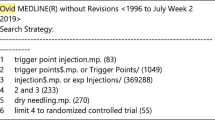
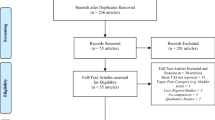
Myofascial pain syndrome (MPS) is a common chronic pain condition that is characterized by distinct “trigger points.” Despite current treatments with physical therapy, analgesics, anti-depressants and trigger-point injections, myofascial pain remains a challenging chronic pain condition in clinical practice. Botulinum toxin A (BTX-A) can cause prolonged muscle relaxation through inhibition of acetylcholine release. It may offer some advantages over the current treatments for MPS by providing a longer sustained period of pain relief. Despite numerous clinical trials, the efficacy of BTX-A in alleviating MPS is not well-established due to mixed results from recent clinical trials. Active trigger points are associated with referred pain and greatly impact many aspects of activities of daily living, mood, and health status. This review is designed to analyze the clinical trials regarding the efficacy of BTX-A injection of active trigger points as a treatment for MPS. The literature referenced was obtained via a computer search with Google Scholar, Pubmed, Medline and EMbase. Our search terms included “Botulinum toxin,” “myofascial pain,” “trigger points,” “myofascial trigger points,” “chronic pain.” Additional references were retrieved from the reference list of the reports found via this search. Studies were considered eligible for inclusion if they were double-blinded, randomized, controlled trials evaluating the efficacy of BTX-A injections into trigger points for pain reduction, and if the trigger point selection in the trial included referred pain and/or local twitch response. Open-label studies, case reports, and other non-randomized studies were excluded. Eight trials were found according to the above criteria and are summarized in Table 1. There are well-designed clinical trials to support the efficacy of trigger-point injections with BTX-A for MPS. However, further clinical trials with considerations of minimizing placebo effect, repeated dosing, adequate coverage of trigger points, and using ultrasound confirmation and guidance are required to provide conclusive evidence for BTX-A in the treatment of myofascial pain.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Han SC, Harrison P. Myofascial pain syndrome and trigger-point management. Reg Anesth. 1997;22:89–101.
Fricton JR, Kroening R, Haley D, Siegert R. Myofascial pain syndrome of the head and neck: a review of clinical characteristics of 164 patients. Oral Surg Oral Med Oral Pathol. 1985;60:615–23.
Vázquez-Delgado E, Cascos-Romero J, Gay-Escoda C. Myofascial pain associated to trigger points: a literature review. Part 2: Differential diagnosis and treatment. Med Oral Patol Oral Cir Bucal. 2010;15(4):e639–43.
Simons D. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14:95–107.
Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi:10.1016.
Dunne JW, Gracies JM, Hayes M, Zeman B, Singer BJ. A prospective, multicentre, randomized, double-blind, placebo-controlled trial of onabotulinumtoxin A to treat plantarflexor/invertor overactivity after stroke. Clin Rehabil. 2012;26(9):787–97.
McCrory P, Turner-Stokes L, Baguley IJ, De Graaff S, Katrak P, Sandanam J, et al. Botulinum toxin A for treatment of upper limb spasticity following stroke: a multi-center randomized placebo-controlled study of the effects on quality of life and other person-centred outcomes. J Rehabil Med. 2009;41(7):536–44.
Borodic GE, Acquadro M, Johnson EA. Botulinum toxin therapy for pain and inflammatory disorders: mechanisms and therapeutic effects. Expert Opin Investig Drugs. 2001;10:1531–44.
Hallett M. One man’s position: clinical applications of botulinum toxin. N Engl J Med. 1999;341:118–20.
Quintner JL, Cohen ML. Referred pain of peripheral nerve origin:an alternative to the “myofascial pain” construct. Clin J Pain. 1994;10:243–51.
Meng J, Wang J, Lawrence G, Dolly GO. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons to inhibition by botulinum toxins reflects their antinociceptive potential. J Cell Sci. 2007;120:2864–74.
Alo KM, Yland MJ, Kramer DL, Charnov JH, Redko V. Botulinum toxin in the treatment of myofascial pain. The Pain Clinic. 1997;10:107–16.
De Andrés J, Cerda-Olmedo G, Valía JC, Monsalve V, Lopez-Alarcón, Minguez A. Use of botulinum toxin in the treatment of chronic myofascial pain. Clin J Pain. 2003;19(4):269–75.
Arezzo JC. Possible mechanisms for the effects of botulinum toxin on pain. Clin J Pain. 2002;18(6 Suppl):S125–32.
Gazerani P et al. Botulinum toxin A decreases the mechanical sensitivity of nociceptors and inhibits neurogenic vasodilation in a craniofacial. Pain. 2010;151(3):606–16.
Lucion A, Bales GT, Lotan LT, et al. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008;101:366–70.
Bach-Rojecky L, Lackovic Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Bihav. 2009;94:234–8.
Pearse JMS. Myofascial pain, fibromyalgia or fibrositis? Eur Neurol. 2004;52:67–72.
Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653–60.
Hong CZ. Pathophysiology of myofascial trigger point. J Formos Med Assoc. 1996;95(2):93–104.
Gerber LH, Sikdar S, Armstrong K, Diao G, Heimur J, Kopecky J et al. A systematic comparison between subjects with no pain and pain associated with active myofascial trigger points. PM&R. 2013;5(11):931–8.
Munns M, Hughes A. Botulinum toxin A for treatment of upper limb spasticity following stroke: a multi-centre randomized placebo-controlled study of the effects on quality of life and other person-centred outcomes. J Rehabil Med. 2009;41(7):536–44.
Simons DG, Travell JG, Simons LS, Travell JG. Travell & Simons’myofascial pain and dysfunction the trigger point manual. 2nd ed. Baltimore: Williams & Wilkins; 1999.
Gobel H, Heinze A, Reichel G, Hefter H, Benecke R. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomized double-blind placebo-controlled multicentre study. Pain. 2006;125:82–8. An important study with ten Botulinum Toxin A trigger point injections for each patient. The study showed statistically significant improvement compared with placebo.
Freund BJ, Schwartz M. Treatment of chronic cervical-associated headache with botulinum toxin A: a pilot study. Headache. 2000;40:231–6.
Cheshire WP, Abashian SW, Mann JD. Botulinum toxin in the treatment of myofascial pain syndrome. Pain. 1994;59(1):65–9.
Ferrante FM, Bearn L, Rothrock R, King L. Evidence against trigger point injection technique for the treatment of cervicothoracic myofascial pain with botulinum toxin type A. Anesthesiology. 2005;103:377–83.
Wheeler AH, Goolkasian P, Gretz SS. Botulinum toxin A for the treatment of chronic neck pain. Pain. 2001;94(3):255–60.
Graboski CL, Gray DS, Burnham RS. Botulinum toxin A versus bupivacaine trigger point injections for the treatment of myofascial pain syndrome: a randomized double blind crossover study. Pain. 2005;118:170–5.
Wheeler AH, Goolkasian P, Gretz SS. A randomized, double-blind, prospective pilot study of botulinum toxin injection for refractory, unilateral, cervicothoracic, paraspinal, myofascial pain syndrome. Spine (Phila Pa 1976). 1998;23(15):1662–6.
Qerama E, Fuglsang-Frederiksen A, Kasch H, Bach FW, Jensen TS. A double-blind, controlled study of botulinum toxin A in chronic myofascial pain. Neurology. 2006;67:241–5.
Benecke R, Heinze A, Reichel G, Hefter H, Göbel H. Botulinum type A toxin complex for the relief of back myofascial pain syndrome: how do fixed-location injections compare with trigger point-focused injections? Pain Medicine. 2011;12:1607–14. This study is the first to use a set of standardized trigger point sites that included ten predetermined fixed trigger point injections in the head, neck, and shoulder. This study found significant improvement at eight weeks after treatment with BTX-A for patients with upper back myofascial pain syndrome.
Cummings TM, White AR. Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch Phys Med Rehabil. 2001;82:986–92.
Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J Ultrasound Med. 2011;30(10):1331–40. A well-designed study reported Ultrasound can objectively identify active trigger points.
Simons DG. Clinical and etiological update of myofascial pain from trigger points. J Musculoskelet Pain. 1996;4(1/2):93–121.
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Jon Y. Zhou and Dr. Dajie Wang reported no potential conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Anesthetic Techniques in Pain Management
Rights and permissions
About this article
Cite this article
Zhou, J.Y., Wang, D. An Update on Botulinum Toxin A Injections of Trigger Points for Myofascial Pain. Curr Pain Headache Rep 18, 386 (2014). https://doi.org/10.1007/s11916-013-0386-z
Published:
DOI: https://doi.org/10.1007/s11916-013-0386-z