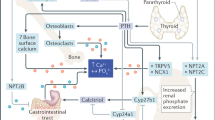
Abstract
Purpose of review
This review summarizes recently published data on the effects of pregnancy and lactation on bone structure, mechanical properties, and mechano-responsiveness in an effort to elucidate how the balance between the structural and metabolic functions of the skeleton is achieved during these physiological processes.
Recent findings
While pregnancy and lactation induce significant changes in bone density and structure to provide calcium for fetal/infant growth, the maternal physiology also comprises several innate compensatory mechanisms that allow for the maintenance of skeletal mechanical integrity. Both clinical and animal studies suggest that pregnancy and lactation lead to adaptations in cortical bone structure to allow for rapid calcium release from the trabecular compartment while maintaining whole bone stiffness and strength. Moreover, extents of lactation-induced bone loss and weaning-induced recovery are highly dependent on a given bone’s load-bearing function, resulting in better protection of the mechanical integrity at critical load-bearing sites. The recent discovery of lactation-induced osteocytic perilacunar/canalicular remodeling (PLR) indicates a new means for osteocytes to modulate mineral homeostasis and tissue-level mechanical properties of the maternal skeleton. Furthermore, lactation-induced PLR may also play an important role in maintaining the maternal skeleton’s load-bearing capacity by altering osteocyte’s microenvironment and modulating the transmission of anabolic mechanical signals to osteocytes.
Summary
Both clinical and animal studies show that parity and lactation have no adverse, or a positive effect on bone strength later in life. The skeletal effects during pregnancy and lactation reflect an optimized balance between the mechanical and metabolic functions of the skeleton.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
2017 Global health observatory data. World Health Organization.
Horta BL, Victora CG. Long-term effects of breastfeeding. World Health Organization; 2013.
Global Nutrition Targets 2025: Breastfeeding policy brief. World Health Organization, UNICEF; 2014.
• Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev. 2016;96(2):449–547 This paper provided a comprehensive review on skeletal and mineral physiology of the maternal skeleton and disorders of bone and mineral metabolism during pregnancy, lactation, and post-weaning recovery.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8.
Moller UK, Vieth Streym S, Mosekilde L, Rejnmark L. Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos Int. 2012;23(4):1213–23.
Kaur M, Pearson D, Godber I, Lawson N, Baker P, Hosking D. Longitudinal changes in bone mineral density during normal pregnancy. Bone. 2003;32(4):449–54.
Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res. 2000;15(3):557–63.
Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res. 2000;15(1):129–37.
Honda A, Kurabayashi T, Yahata T, Tomita M, Takakuwa K, Tanaka K. Lumbar bone mineral density changes during pregnancy and lactation. Int J Gynaecol Obstet. 1998;63(3):253–8.
Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. N Engl J Med. 1997;337(8):523–8.
Karlsson C, Obrant KJ, Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int. 2001;12(10):828–34.
Sowers M, Corton G, Shapiro B, Jannausch ML, Crutchfield M, Smith ML, et al. Changes in bone density with lactation. Jama. 1993;269(24):3130–5.
Recker R, Lappe J, Davies K, Heaney R. Characterization of perimenopausal bone loss: a prospective study.[see comment]. J Bone Miner Res. 2000;15(10):1965–73.
Collins JN, Kirby BJ, Woodrow JP, Gagel RF, Rosen CJ, Sims NA, et al. Lactating Ctcgrp nulls lose twice the normal bone mineral content due to fewer osteoblasts and more osteoclasts, whereas bone mass is fully restored after weaning in association with up-regulation of Wnt signaling and other novel genes. Endocrinology. 2013;154(4):1400–13.
VanHouten JN, Wysolmerski JJ. Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology. 2003;144(12):5521–9.
Honda A, Kurabayashi T, Yahata T, Tomita M, Matsushita H, Takakuwa K, et al. Effects of pregnancy and lactation on trabecular bone and marrow adipocytes in rats. Calcif Tissue Int. 2000;67(5):367–72.
• de Bakker CMJ, Tseng WJ, Li Y, Zhao H, Altman-Singles AR, Jeong Y, et al. Reproduction differentially affects trabecular bone depending on its mechanical versus metabolic role. J Biomech Eng. 2017;139(11) This study quantified the proportion of the load carried by the trabeculae, as well as the extent of reproductive loss and recovery in bone volume, structure, cellular activities, and mechanical properties, at two distinct skeletal sites: the tibia and lumbar vertebra. The differential trabecular response indicate differences in the extent of the trabecular bone’s structural vs. metabolic functions at these two locations.
Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27(5):1018–29.
Jahn K, Kelkar S, Zhao H, Xie Y, Tiede-Lewis LM, Dusevich V, et al. Osteocytes acidify their microenvironment in response to PTHrP in vitro and in lactating mice in vivo. J Bone Miner Res. 2017;32(8):1761–72.
• Kaya S, Basta-Pljakic J, Seref-Ferlengez Z, Majeska RJ, Cardoso L, Bromage TG, et al. Lactation-induced changes in the volume of osteocyte lacunar-canalicular space alter mechanical properties in cortical bone tissue. J Bone Miner Res. 2017;32(4):688–97 This study demonstrates that tissue-level cortical bone mechanical properties are rapidly and reversibly modulated by osteocytes in response to lactation and weaning.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38.
Gordon MC. Maternal Physiology. Obstetrics: normal and problem pregnancies. 2012.
VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, et al. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest. 2003;112(9):1429–36.
Ardeshirpour L, Brian S, Dann P, VanHouten J, Wysolmerski J. Increased PTHrP and decreased estrogens alter bone turnover but do not reproduce the full effects of lactation on the skeleton. Endocrinology. 2010;151(12):5591–601.
Seriwatanachai D, Thongchote K, Charoenphandhu N, Pandaranandaka J, Tudpor K, Teerapornpuntakit J, et al. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone. 2008;42(3):535–46.
Bornstein S, Brown SA, Le PT, Wang X, DeMambro V, Horowitz MC, et al. FGF-21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology. 2014;155(9):3516–26.
Lippuner K, Zehnder HJ, Casez JP, Takkinen R, Jaeger P. PTH-related protein is released into the mother’s bloodstream during lactation: evidence for beneficial effects on maternal calcium-phosphate metabolism. J Bone Miner Res. 1996;11(10):1394–9.
Ardeshirpour L, Dann P, Adams DJ, Nelson T, VanHouten J, Horowitz MC, et al. Weaning triggers a decrease in receptor activator of nuclear factor-kappaB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology. 2007;148(8):3875–86.
Miller SC, Bowman BM. Rapid inactivation and apoptosis of osteoclasts in the maternal skeleton during the bone remodeling reversal at the end of lactation. Anat Rec (Hoboken). 2007;290(1):65–73.
Bowman BM, Siska CC, Miller SC. Greatly increased cancellous bone formation with rapid improvements in bone structure in the rat maternal skeleton after lactation. J Bone Miner Res. 2002;17(11):1954–60.
Miller SC, Anderson BL, Bowman BM. Weaning initiates a rapid and powerful anabolic phase in the rat maternal skeleton. Biol Reprod. 2005;73(1):156–62.
Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7(2):137–45.
Cundy T, Cornish J, Evans MC, Gamble G, Stapleton J, Reid IR. Sources of interracial variation in bone mineral density. J Bone Miner Res. 1995;10(3):368–73.
Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73(6):1332–9.
Goulet RW, Goldstein SA, Ciarelli MJ, Kuhn JL, Brown MB, Feldkamp LA. The relationship between the structural and orthogonal compressive properties of trabecular bone. J Biomech. 1994;27(4):375–89.
Keaveny TM, Morgan EF, Niebur GL, Yeh OC. Biomechanics of trabecular bone. Annu Rev Biomed Eng. 2001;3:307–33.
Kim HS, Al-Hassani ST. A morphological model of vertebral trabecular bone. J Biomech. 2002;35(8):1101–14.
Van Rietbergen B, Odgaard A, Kabel J, Huiskes R. Relationships between bone morphology and bone elastic properties can be accurately quantified using high-resolution computer reconstructions. J Orthop Res. 1998;16(1):23–8.
Hellmeyer L, Hahn B, Fischer C, Hars O, Boekhoff J, Maier J, et al. Quantitative ultrasonometry during pregnancy and lactation: a longitudinal study. Osteoporos Int. 2015;26(3):1147–54.
Della Martina M, Biasioli A, Vascotto L, Rinuncini D, Adorati Menegato A, Liva S, et al. Bone ultrasonometry measurements during pregnancy. Arch Gynecol Obstet. 2010;281(3):401–7.
Korecki CL, Zinser G, Liu X, Siedler J, Welsh J, Niebur GL. Effect of the vitamin D receptor on bone geometry and strength during gestation and lactation in mice. Calcif Tissue Int. 2009;85(5):405–11.
Vajda EG, Bowman BM, Miller SC. Cancellous and cortical bone mechanical properties and tissue dynamics during pregnancy, lactation, and postlactation in the rat. Biol Reprod. 2001;65(3):689–95.
• de Bakker CM, Altman-Singles AR, Li Y, Tseng WJ, Li C, Liu XS. Adaptations in the Microarchitecture and load distribution of maternal cortical and trabecular bone in response to multiple reproductive cycles in rats. J Bone Miner Res. 2017;32(5):1014–26 This study longitudinally tracked the bone structural changes in rat proximal tibia in response to 3 reproductive cycles and demonstrated that pregnancy and lactation resulted in both transient and long-lasting alterations in trabecular microstructure, improvement in the robustness of cortical bone, and increased proportion of the total load carried by the cortical bone, allowing the overall mechanical function of the tibia to be maintained.
Kovacs CS, Ralston SH. Presentation and management of osteoporosis presenting in association with pregnancy or lactation. Osteoporos Int. 2015;26(9):2223–41.
Laskey MA, Price RI, Khoo BC, Prentice A. Proximal femur structural geometry changes during and following lactation. Bone. 2011;48(4):755–9.
To WW, Wong MW. Changes in bone mineral density of the os calcis as measured by quantitative ultrasound during pregnancy and 24 months after delivery. Aust N Z J Obstet Gynaecol. 2011;51(2):166–71.
Yumusakhuylu Y, Turgut ST, Icagasioglu A, Baklacioglu HS, Atlig RS, Murat S, et al. Bone mineral changes during pregnancy and lactation. Gynecol Endocrinol. 2013;29(8):763–6.
Brembeck P, Lorentzon M, Ohlsson C, Winkvist A, Augustin H. Changes in cortical volumetric bone mineral density and thickness, and trabecular thickness in lactating women postpartum. J Clin Endocrinol Metab. 2015;100(2):535–43.
Bjornerem A, Ghasem-Zadeh A, Wang X, Bui M, Walker SP, Zebaze R, et al. Irreversible deterioration of cortical and trabecular microstructure associated with breastfeeding. J Bone Miner Res. 2017;32(4):681–7.
Brembeck P, Winkvist A, Ohlsson C, Lorentzon M, Augustin H. Determinants of microstructural, dimensional and bone mineral changes postpartum in Swedish women. Br J Nutr. 2016:1–9.
Kokolus S, Vrabel MB, Liu XS, Boutroy S, Rogers H, McMahon D, et al. High resolution peripheral quantitative CT (HRpQCT) reveals preferential inner trabecular bone loss in lactating women. American Society for Bone and Mineral Research Annual Meeting; 2010; Toronto. Canada. .
Kepley A, Boutroy S, Zhang C, Bucovsky M, Vrabel MB, Kokolus S, et al. In breastfeeding women, trabecular bone loss at the radius, seen by high resolution peripheral quantitative CT (HRpQCT), persists at 18 months postpartum. American Society of Bone and Mineral Research Annual Meeting; 2012; Minneapolis, MN.
Liu XS, Ardeshirpour L, VanHouten JN, Shane E, Wysolmerski JJ. Site-specific changes in bone microarchitecture, mineralization, and stiffness during lactation and after weaning in mice. J Bone Miner Res. 2012;27(4):865–75.
Ross RD, Meagher MJ, Sumner DR. Calcium restriction during lactation has minimal effects on post-weaning mineral metabolism and bone recovery. J Bone Miner Metab. 2019;37(4):648–57.
Gillies BR, Ryan BA, Tonkin BA, Poulton IJ, Ma Y, Kirby BJ, et al. Absence of calcitriol causes increased lactational bone loss and lower milk calcium but does not impair post-lactation bone recovery in Cyp27b1 null mice. J Bone Miner Res. 2018;33(1):16–26.
Wiklund PK, Xu L, Wang Q, Mikkola T, Lyytikainen A, Volgyi E, et al. Lactation is associated with greater maternal bone size and bone strength later in life. Osteoporos Int. 2012;23(7):1939–45.
Specker B, Binkley T. High parity is associated with increased bone size and strength. Osteoporos Int. 2005;16(12):1969–74.
de Bakker CMJ, Zhao H, Tseng WJ, Li Y, Altman-Singles AR, Liu Y, et al. Effects of reproduction on sexual dimorphisms in rat bone mechanics. J Biomech. 2018.
Pearson D, Kaur M, San P, Lawson N, Baker P, Hosking D. Recovery of pregnancy mediated bone loss during lactation. Bone. 2004;34(3):570–8.
Whitehead CC. Overview of bone biology in the egg-laying hen. Poult Sci. 2004;83(2):193–9.
ACOG Committee Opinion No. 650: Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 2015;126(6):e135–42.
Dimov M, Khoury J, Tsang R. Bone mineral loss during pregnancy: is tennis protective? J Phys Act Health. 2010;7(2):239–45.
To WW, Wong MW. Bone mineral density changes during pregnancy in actively exercising women as measured by quantitative ultrasound. Arch Gynecol Obstet. 2012;286(2):357–63.
Promislow JH, Hertz-Picciotto I, Schramm M, Watt-Morse M, Anderson JJ. Bed rest and other determinants of bone loss during pregnancy. Am J Obstet Gynecol. 2004;191(4):1077–83.
Lovelady CA, Bopp MJ, Colleran HL, Mackie HK, Wideman L. Effect of exercise training on loss of bone mineral density during lactation. Med Sci Sports Exerc. 2009;41(10):1902–7.
Little KD, Clapp JF 3rd. Self-selected recreational exercise has no impact on early postpartum lactation-induced bone loss. Med Sci Sports Exerc. 1998;30(6):831–6.
Hemmatian H, Jalali R, Semeins CM, Hogervorst JMA, van Lenthe GH, Klein-Nulend J, et al. Mechanical loading differentially affects osteocytes in fibulae from lactating mice compared to osteocytes in virgin mice: possible role for lacuna size. Calcif Tissue Int. 2018;103(6):675–85.
Rosa BV, Blair HT, Vickers MH, Morel PC, Cockrem JF, Firth EC. Voluntary exercise in pregnant rats improves post-lactation maternal bone parameters but does not affect offspring outcomes in early life. J Musculoskelet Neuronal Interact. 2012;12(4):199–208.
Shea JE, Doody SL, Elsenman PA, Miller SC, editors. Augmentation of bone gains during the anabolic post-lactation recovery period utilizing a rat bipedal stance model. American Society of Bone and Mineral Research Annual Meeting; 2005; Nashville, TN.
• Tsourdi E, Jahn K, Rauner M, Busse B, Bonewald LF. Physiological and pathological osteocytic osteolysis. J Musculoskelet Neuronal Interact. 2018;18(3):292–303 This paper provided a comprehensive review on the peri-lacunar/canalicular remodeling in physiological and pathological conditions and the current understanding of molecular mechanisms behind this osteocyte function.
Yee CS, Schurman CA, White CR, Alliston T. Investigating osteocytic perilacunar/canalicular remodeling. Cur Osteopor Rep. 2019;17(4):157–68.
Tang SY, Herber RP, Ho SP, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012;27(9):1936–50.
Wang L. Solute transport in the bone lacunar-canalicular system (LCS). Cur Osteopor Rep. 2018;16(1):32–41.
Fritton SP, Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech. 2009;41:347–74.
• Wang B, Lai X, Price C, Thompson WR, Li W, Quabili TR, et al. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2014;29(4):878–91 This study linked cellular hydaulic forces (shear and drag) with in vivo bone formation and provided evidence that osteocyte pericellular matrix serves as a flow sensor in the lacunar-canalicular system.
• Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27(3):339–60 This paper established the theoritical framework demonstrating interstitial fluid flow in mechanically loaded bone imparts fluid shear stress on the osteocyte cell process membrane.
Price C, Zhou X, Li W, Wang L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J Bone Miner Res. 2011;26(2):277–85.
Wang B, Zhou X, Price C, Li W, Pan J, Wang L. Quantifying load-induced solute transport and solute-matrix interaction within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2013;28(5):1075–86.
You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34(11):1375–86.
You LD, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol. 2004;278(2):505–13.
Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104(33):13325–30.
Thompson WR, Modla S, Grindel BJ, Czymmek KJ, Kirn-Safran CB, Wang L, et al. Perlecan/Hspg2 deficiency alters the pericellular space of the lacunocanalicular system surrounding osteocytic processes in cortical bone. J Bone Miner Res. 2011;26(3):618–29.
Wijeratne SS, Martinez JR, Grindel BJ, Frey EW, Li J, Wang L, et al. Single molecule force measurements of perlecan/HSPG2: a key component of the osteocyte pericellular matrix. Matrix Biol. 2016;50:27–38.
Wysolmerski JJ. Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Ann N Y Acad Sci. 2010;1192:161–9.
Klein-Nulend J, van Oers RF, Bakker AD, Bacabac RG. Bone cell mechanosensitivity, estrogen deficiency, and osteoporosis. J Biomech. 2015;48(5):855–65.
Rooney AM, van der Meulen MCH. Mouse models to evaluate the role of estrogen receptor alpha in skeletal maintenance and adaptation. Ann N Y Acad Sci. 2017;1410(1):85–92.
Esbrit P, Herrera S, Portal-Nunez S, Nogues X, Diez-Perez A. Parathyroid hormone-related protein analogs as osteoporosis therapies. Calcif Tissue Int. 2016;98(4):359–69.
Li Y, De Bakker CM, Tseng WJ, Zhao H, Parajuli A, Wang L et al. Peri-lacunar/canalicular (PLC) remodeling enhances mechano-sensitivity in rat maternal bone when subjected to estrogen deficiency. American Society of Bone and Mineral Research Annual Meeting; 2018; Montreal, Canada.
Funding
This work was supported by NIH K01-AR066743 (to XSL), NIH R01-AR071718 (to XSL), NIH R01-AR054385 (to LW), and NSF #1653216 (to XSL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
X. Sherry Liu, Liyun Wang, Chantal M. J. de Bakker, and Xiaohan Lai declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomechanics
Rights and permissions
About this article
Cite this article
Liu, X.S., Wang, L., de Bakker, C.M.J. et al. Mechanical Regulation of the Maternal Skeleton during Reproduction and Lactation. Curr Osteoporos Rep 17, 375–386 (2019). https://doi.org/10.1007/s11914-019-00555-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-019-00555-5