Abstract
Hepatitis B infection is best controlled by prevention rather than treatment, as chronic infection is usual once infected at a young age. Infant immune-prophylaxis is highly efficacious, although in the setting of high maternal viral load, breakthrough infection still occurs in almost 10 % of babies. Ante partum antiviral therapy for the purpose of preventing mother to child transmission in this group is important to consider. This article provides an up-to-date account of the available evidence of the safety and efficacy of ante partum antiviral therapy options and a management plan is proposed. Mothers with HBV infection and high viral load should have the opportunity to consider this evidence. Post-partum HBV flares are common but usually mild. It is unclear whether post partum flares are best ignored till they settle or if they represent an opportunity for intervention to increase the chance of HBV clearance.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
O’Sullivan BG, Gidding HF, Law M, Kaldor JM, Gilbert GL, Dore GJ. Estimates of chronic hepatitis B virus infection in Australia, 2000. Aust N Z J Public Health. 2004;28:212–6.
Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol. 1977;105:94–8.
del Canho R, Grosheide PM, Mazel JA, Heijtink RA, Hop WC, Gerards LJ, de Gast GC, et al. Ten-year neonatal hepatitis B vaccination program, The Netherlands, 1982–1992: protective efficacy and long-term immunogenicity. Vaccine. 1997;15:1624–30.
Chen D-S. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50:805–16.
Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992–1000.
Senise JF, Castelo A, Martinez M. Current treatment strategies, complications and considerations for the use of HIV antiretroviral therapy during pregnancy. AIDS Rev. 2011;13:198–213.
European Association For The Study Of The L. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–42.
Petersen J. HBV treatment and pregnancy. J Hepatol. 2011;55:1171–3.
Tran TT. Management of hepatitis B in pregnancy: weighing the options. Cleve Clin J Med. 2009;76 Suppl 3:S25–9.
Song Y-M, Sung J, Yang S, Choe YH, Chang YS, Park WS. Factors associated with immunoprophylaxis failure against vertical transmission of hepatitis B virus. Eur J Pediatr. 2007;166:813–8.
van Zonneveld M, van Nunen AB, Niesters HGM, de Man RA, Schalm SW, Janssen HLA. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10:294–7.
Shi Z, Yang Y, Ma L, Li X, Schreiber A. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147–59.
• Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19:e18–25. This study retrospectively examined the virological factors assocaited with failure to passive-active immunoprphylaxis in infacnts born to HBsAg postive mothers and foudn that viral load was the most signfiicant. Of note all failure infants were born to HBeAg postiive mothers.
Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, Huang FY, Chen CL. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099–102.
• Wiseman E, Fraser MA, Holden S, Glass A, Kidson BL, Heron LG, Maley MW, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190:489–92. This study provided evidence of immunoprophylaxis failure in a western hospital setting, at a similar rates as noted in a large chinese cohort. Transmission was only seen from mothers with viral loads ≥ log 8 copies/ml.
Beasley RP, Stevens CE, Shiao IS, Meng HC. Evidence against breast-feeding as a mechanism for vertical transmission of hepatitis B. Lancet. 1975;2:740–1.
•• Han G-R, Cao M-K, Zhao W, Jiang H-X, Wang C-M, Bai S-F, Yue X, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55:1215–21. This large case control study showed that telbivuine 600 mg started from week 20–32 of pregnancy was highly effective in preventing HBV perinatal tranmsission which continued in the untreated arm Telbivudine was well tolerated.
•• Pan C, Guo-Rong H, Jiang H-X, Zhao W, Cao M-K, Want C-M, Yue X, et al. Telbivudine Prevents Vertical Transmission from HBeAg psotive women with Chroinic Hepatitis B. Clin Gastroenterol Hepatol. 2012;10:520–6. This case control study also showed that telbivdine was effective in preventing perinalt HBV trasmission. This cohort differed from Han et al., in that these mothers had elevated ALT (≥40) at baseline as well as high viral load. The majority continued telbivudine post-partum and unlike the untreated cohort, a signfiicant proportion of these had HBeAg seroconversion.
Bzowej NH. Optimal management of the hepatitis B patient who desires pregnancy or is pregnant. Curr Hepat Rep 2012:11(2)82–89.
Xu WM, Cui YT, Wang L, Yang H, Liang ZQ, Li XM, Zhang SL, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16:94–103.
Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–61.
Lawler J, Glass AL, Chatterjee U, Wiseman E, Davison S, Manoharan S, Smith L, et al. Nucleot(s)ide analogues to prevent perinatal transmission of HBV: Lamivudine is effective but tenofovir may be better. Hepatology. 2011;54s1:1117.
Mandelbrot L, Peytavin G, Firtion G, Farinotti R. Maternal-fetal transfer and amniotic fluid accumulation of lamivudine in human immunodeficiency virus-infected pregnant women. Am J Obstet Gynecol. 2001;184:153–8.
Brown RS, Buti M, Goodwin D, Zhang S, Fagan EA. 318 Hepatitis B Virus (HBV) Drugs in Pregnancy: findings from the Antiretroviral Pregnancy Registry. Gastroenterology. 2009;136:A-798.
Committee. APRS. Antiretroviral pregnancy registry international interim repport for 1 Jan 1989 through 31 July 2008. <http://www.apregistry.com/forms/interim_report.pdf>. In.
Giles M, Visvanathan K, Sasadeusz J. Antiviral therapy for hepatitis B infection during pregnancy and breastfeeding. Antivir Ther. 2011;16:621–8.
Chappuy H, Treluyer J-M, Jullien V, Dimet J, Rey E, Fouche M, Firtion G, et al. Maternal-fetal transfer and amniotic fluid accumulation of nucleoside analogue reverse transcriptase inhibitors in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2004;48:4332–6.
Mirochnick M, Thomas T, Capparelli E, Zeh C, Holland D, Masaba R, Odhiambo P, et al. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2009;53:1170–6.
Moodley J, Moodley D, Pillay K, Coovadia H, Saba J, van Leeuwen R, Goodwin C, et al. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J Infect Dis. 1998;178:1327–33.
Buster EHCJ, van Erpecum KJ, Schalm SW, Zaaijer HL, Brouwer JT, Gelderblom HC, de Knegt RJ, et al. Treatment of chronic hepatitis B virus infection—Dutch national guidelines. Neth J Med. 2008;66:292–306.
Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology 2009;137:1593–1608.e1591-1592.
Villet S, Pichoud C, Billioud G, Barraud L, Durantel S, Trepo C, Zoulim F. Impact of hepatitis B virus rtA181V/T mutants on hepatitis B treatment failure. J Hepatol. 2008;48:747–55.
Paik YH, Chung HY, Ryu WS, Lee KS, Lee JS, Kim JH, Lee CK, et al. Emergence of YMDD motif mutant of hepatitis B virus during short-term lamivudine therapy in South Korea. J Hepatol. 2001;35:92–8.
Ayres A, Yuen L, Manoharan S, Glass A, Maley M, Levy M, Jackson k, et al. Ultradeep pyrosequencing identifies multidrug resistant HBV in pregnancy women undergoing lamivudine therapy. Hepatology 2011 October; 1074A.
Gane EJ, Wang Y, Liaw Y-F, Hou J, Thongsawat S, Wan M, Moon YM, et al. Efficacy and safety of prolonged 3-year telbivudine treatment in patients with chronic hepatitis B. Liver Int. 2011;31:676–84.
Liaw Y-F, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, Heathcote EJ, et al. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486–95.
Zeuzem S, Gane E, Liaw Y-F, Lim SG, DiBisceglie A, Buti M, Chutaputti A, et al. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol. 2009;51:11–20.
Tarantal AF, Castillo A, Ekert JE, Bischofberger N, Martin RB. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta). J Acquir Immune Defic Syndr JAIDS. 2002;29:207–20.
Van Rompay KKA, Brignolo LL, Meyer DJ, Jerome C, Tarara R, Spinner A, Hamilton M, et al. Biological effects of short-term or prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (tenofovir) to newborn and infant rhesus macaques. [Erratum appears in Antimicrob Agents Chemother. 2994 Jan;48(6):2346]. Antimicrob Agents Chemother. 2004;48:1469–87.
Van Rompay KKA, Durand-Gasselin L, Brignolo LL, Ray AS, Abel K, Cihlar T, Spinner A, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother. 2008;52:3144–60.
•• Siberry GK, Williams PL, Mendez H, Seage III GR, Jacobson DL, Hazra R, Rich KC, et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26:1151–9. This study examined the effect tenofovir as part of anti retroviral use in pregnancy on the growth and development of 449 infants, as assessed at term and at 1 year. Reassuring information was provided at term, with no difference in growth measurements when tenofovir was used although at 1 year a tenofovir exposed infants were 32 mm shorter and had a 41 mm smaller head circumference. The significance of thisfinding at 12 months is uncertain.
Nurutdinova D, Onen NF, Hayes E, Mondy K, Overton ET. Adverse effects of tenofovir use in HIV-infected pregnant women and their infants. Ann Pharmacother. 2008;42:1581–5.
Benhammou V, Warszawski J, Bellec S, Doz F, Andre N, Lacour B, Levine M, et al. Incidence of cancer in children perinatally exposed to nucleoside reverse transcriptase inhibitors. AIDS. 2008;22:2165–77.
Giacomet V, Mora S, Martelli L, Merlo M, Sciannamblo M, Vigano A. A 12-month treatment with tenofovir does not impair bone mineral accrual in HIV-infected children. J AcquirImmune Defic Syndr JAIDS. 2005;40:448–50.
Mora S, Giacomet V, Vigano A, Cafarelli L, Stucchi S, Pivetti V, Manfredini V, et al. Exposure to antiretroviral agents during pregnancy does not alter bone status in infants. Bone. 2012;50:255–8.
Van Rompay KKA, Hamilton M, Kearney B, Bischofberger N. Pharmacokinetics of tenofovir in breast milk of lactating rhesus macaques. Antimicrob Agents Chemother. 2005;49:2093–4.
Benaboud S, Hirt D, Launay O, Pannier E, Firtion G, Rey E, Bouazza N, et al. Pregnancy-related effects on tenofovir pharmacokinetics: a population study with 186 women. Antimicrob Agents Chemother. 2012;56:857–62.
Tan H, Lui H, Chow W. Chronic hepatitis B virus infection in pregnancy. Hepatol Int. 2008;2:370–5.
Nguyen G, Garcia RT, Nguyen N, Trinh H, Keeffe EB, Nguyen MH. Clinical course of hepatitis B virus infection during pregnancy. Aliment Pharmacol Ther. 2009;29:755–64.
Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledee N. Tolerance to the foetal allograft? Am J Reprod Immunol. 2010;63:624–36.
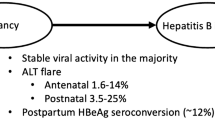
Lin HH, Chen PJ, Chen DS, Sung JL, Yang KH, Young YC, Liou YS, et al. Postpartum subsidence of hepatitis B viral replication in HBeAg-positive carrier mothers. J Med Virol. 1989;29:1–6.
Lin H-H, Wu W-Y, Kao J-H, Chen D-S. Hepatitis B post-partum e antigen clearance in hepatitis B carrier mothers: correlation with viral characteristics. J Gastroenterol Hepatol. 2006;21:605–9.
ter Borg MJ, Leemans WF, de Man RA, Janssen HLA. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat. 2008;15:37–41.
Tan PK, Chatterjee U, Glass AL, Maley M, Ayres A, Locarnini S, Levy M. Lamivudine in pregnancy: impact on Hepatitis B flares and HBeAg seroconversion post partum. Hepatology 2012. (in press)
Disclosure
No potential conflict of interest relevant to this article was reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levy, M.T. Preventing Perinatal Transmission of HBV: An Australian Perspective. Curr Hepatitis Rep 11, 206–212 (2012). https://doi.org/10.1007/s11901-012-0144-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-012-0144-4