Abstract
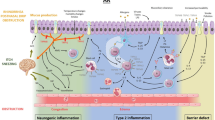
Sensory nerve endings within the airway epithelial cells and the solitary chemoreceptor cells, synapsing with sensory nerves, respond to airborne irritants. Transient receptor potential (TRP) channels (A1 and V1 subtypes, specifically) on these nerve endings initiate local antidromic reflexes resulting in the release of neuropeptides such as substance P and calcitonin G-related peptides. These neuropeptides dilate epithelial submucosal blood vessels and may therefore increase transudation across these vessels resulting in submucosal edema, congestion, and rhinitis. Altered expression or activity of these TRP channels can therefore influence responsiveness to irritants. Besides these pathogenic mechanisms, additional mechanisms such as dysautonomia resulting in diminished sympathetic activity and comparative parasympathetic overactivity have also been suggested as a probable mechanism. Therapeutic effectiveness for this condition has been demonstrated through desensitization of TRPV1 channels with typical agonists such as capsaicin. Other agents effective in treating nonallergic rhinitis (NAR) such as azelastine have been demonstrated to exhibit TRPV1 channel activity through the modulation of Ca2+ signaling on sensory neurons and in nasal epithelial cells. Roles of antimuscarinic agents such as tiotropium in NAR have been suggested by associations of muscarinic cholinergic receptors with TRPV1. The associations between these channels have also been suggested as mechanisms of airway hyperreactivity in asthma. The concept of the united airway disease hypothesis suggests a significant association between rhinitis and asthma. This concept is supported by the development of late-onset asthma in about 10–40 % of NAR patients who also exhibit a greater severity in their asthma. The factors and mechanisms associating NAR with nonallergic asthma are currently unknown. Nonetheless, free immunoglobulin light chains and microRNA alteration as mediators of these inflammatory conditions may play key roles in this association.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bernstein JA. Characteristics of nonallergic vasomotor rhinitis. World Allergy Organ J. 2009;2(6):102–5. doi:10.1097/WOX.0b013e3181a8e389.
Corren J, Kachru R. Relationship between nonallergic upper airway disease and asthma. Clin Allergy Immunol. 2007;19:101–14.
Settipane RA, Lieberman P. Update on nonallergic rhinitis. Ann Allergy Asthma Immunol. 2001;86(5):494–507. quiz -8. doi:10.1016/S1081-1206(10)62896-7.
Smith TL. Vasomotor rhinitis is not a wastebasket diagnosis. Arch Otolaryngol Head Neck Surg. 2003;129(5):584–7. doi:10.1001/archotol.129.5.584.
Brandt D, Bernstein JA. Questionnaire evaluation and risk factor identification for nonallergic vasomotor rhinitis. Ann Allergy Asthma Immunol. 2006;96(4):526–32. doi:10.1016/S1081-1206(10)63546-6.
Payne SC, Chen PG, Borish L. Local class switching in nonallergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2011;19(3):193–8. doi:10.1097/MOO.0b013e328345005c.
Shusterman D. Environmental nonallergic rhinitis. Clin Allergy Immunol. 2007;19:249–66.
Biedlingmaier JF, Trifillis A. Comparison of CT scan and electron microscopic findings on endoscopically harvested middle turbinates. Otolaryngol Head Neck Surg. 1998;118(2):165–73.
Berger G, Moroz A, Marom Z, Ophir D. Inferior turbinate goblet cell secretion in patients with perennial allergic and nonallergic rhinitis. Am J Rhinol. 1999;13(6):473–7.
Berger G, Marom Z, Ophir D. Goblet cell density of the inferior turbinates in patients with perennial allergic and nonallergic rhinitis. Am J Rhinol. 1997;11(3):233–6.
Drake-Lee A, Ruckley R, Parker A. Occupational rhinitis: a poorly diagnosed condition. J Laryngol Otol. 2002;116(8):580–5. doi:10.1258/00222150260171533.
Baumgarten CR, Togias AG, Naclerio RM, Lichtenstein LM, Norman PS, Proud D. Influx of kininogens into nasal secretions after antigen challenge of allergic individuals. J Clin Invest. 1985;76(1):191–7. doi:10.1172/JCI111945.
Lim MC, Taylor RM, Naclerio RM. The histology of allergic rhinitis and its comparison to cellular changes in nasal lavage. Am J Respir Crit Care Med. 1995;151(1):136–44. doi:10.1164/ajrccm.151.1.7812543.
Bernstein J, Smith A. Physiology and host immune responses of the nose and sinuses. In: Chang CC, Incaudo GA, Gershwin ME, editors. Diseases of the sinuses. New York: Springer; 2014. p. 45–56. Discusses the basic anatomic, physiologic and immunological background of the sinonasal apparatus.
Evans MJ, Plopper CG. The role of basal cells in adhesion of columnar epithelium to airway basement membrane. Am Rev Respir Dis. 1988;138(2):481–3. doi:10.1164/ajrccm/138.2.481.
Tahamiler R, Yener M, Canakcioglu S. Detection of the nasal cycle in daily activity by remote evaluation of nasal sound. Arch Otolaryngol Head Neck Surg. 2009;135(2):137–42. doi:10.1001/archoto.2008.537.
Hasegawa M, Kern EB. The human nasal cycle. Mayo Clin Proc. 1977;52(1):28–34.
Eccles R. A role for the nasal cycle in respiratory defence. Eur Respir J. 1996;9(2):371–6.
Davies AM, Eccles R. Reciprocal changes in nasal resistance to airflow caused by pressure applied to the axilla. Acta Otolaryngol. 1985;99(1–2):154–9.
Carey SA, Ballinger CA, Plopper CG, McDonald RJ, Bartolucci AA, Postlethwait EM, et al. Persistent rhinitis and epithelial remodeling induced by cyclic ozone exposure in the nasal airways of infant monkeys. Am J Physiol Lung Cell Mol Physiol. 2011;300(2):L242–54. doi:10.1152/ajplung.00177.2010.
Topal E, Bakirtas A, Yilmaz O, Karagol IH, Arslan U, Arga M, et al. Predictive factors to differentiate between allergic and nonallergic rhinitis in children. Int Forum Allergy Rhinol. 2014;4(6):447–52. doi:10.1002/alr.21312.
Vichyanond P, Suratannon C, Lertbunnaphong P, Jirapongsananuruk O, Visitsunthorn N. Clinical characteristics of children with non-allergic rhinitis vs with allergic rhinitis. Asian Pac J Allergy Immunol. 2010;28(4):270–4.
Larsen PL, Tos M, Mogensen C. Nasal glands and goblet cells in chronic hypertrophic rhinitis. Am J Otolaryngol. 1986;7(1):28–33.
Baraniuk JN. Neurogenic mechanisms in rhinosinusitis. Curr Allergy Asthma Rep. 2001;1(3):252–61.
Lung MA. The role of the autonomic nerves in the control of nasal circulation. Biol Signals. 1995;4(3):179–85.
Rezvani M, Brandt D, Bernstein JA, Hastings L, Willwerth J. Investigation of olfactory threshold responses in chronic rhinitis subtypes. Ann Allergy Asthma Immunol. 2007;99(6):571–2. doi:10.1016/S1081-1206(10)60389-4.
Gawlik R, Jawor B, Rogala B, Parzynski S, DuBuske L. Effect of intranasal azelastine on substance P release in perennial nonallergic rhinitis patients. Am J Rhinol Allergy. 2013;27(6):514–6. doi:10.2500/ajra.2013.27.3955. Discusses the possible mechanism of action of Azelastine intranasal spray pertaining to its use in NAR, e.g., reduction of SP release into nasal mucosa.
Segboer CL, Holland CT, Reinartz SM, Terreehorst I, Gevorgyan A, Hellings PW, et al. Nasal hyper-reactivity is a common feature in both allergic and nonallergic rhinitis. Allergy. 2013;68(11):1427–34. doi:10.1111/all.12255. Discusses the differences in nasal hyper-reactivity between AR and NAR patients.
Baraniuk JN, Petrie KN, Le U, Tai CF, Park YJ, Yuta A, et al. Neuropathology in rhinosinusitis. Am J Respir Crit Care Med. 2005;171(1):5–11. doi:10.1164/rccm.200403-357OC.
Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583(Pt 1):175–93. doi:10.1113/jphysiol.2007.133231.
Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118(7):2574–82. doi:10.1172/JCI34886.
Garrison SR, Stucky CL. The dynamic TRPA1 channel: a suitable pharmacological pain target? Curr Pharm Biotechnol. 2011;12(10):1689–97.
Keh SM, Facer P, Yehia A, Sandhu G, Saleh HA, Anand P. The menthol and cold sensation receptor TRPM8 in normal human nasal mucosa and rhinitis. Rhinology. 2011;49(4):453–7. doi:10.4193/Rhino11.089.
Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135(3):271–9. doi:10.1016/j.pain.2007.06.005.
Wolf G. New aspects in the pathogenesis and therapy of hyperreflexive rhinopathy. Laryngol Rhinol Otol (Stuttg). 1988;67(9):438–45.
Holzer P, Wachter C, Heinemann A, Jocic M, Lippe IT, Herbert MK. Sensory nerves, nitric oxide and NANC vasodilatation. Arch Int Pharmacodyn Ther. 1995;329(1):67–79.
Shirasaki H, Asakura K, Narita SI, Kataura A. Expression of substance P (NK1) receptor mRNA in human nose. Acta Otolaryngol. 1998;118(5):717–22.
Bernstein JA, Davis BP, Picard JK, Cooper JP, Zheng S, Levin LS. A randomized, double-blind, parallel trial comparing capsaicin nasal spray with placebo in subjects with a significant component of nonallergic rhinitis. Ann Allergy Asthma Immunol. 2011;107(2):171–8. doi:10.1016/j.anai.2011.05.016. Controlled trial validating role of intranasal capsaicin in improving symptoms in rhinitis subjects with a significant NAR component.
Widdicombe J. The tracheobronchial vasculature: a possible role in asthma. Microcirculation. 1996;3(2):129–41.
Bernstein JA, Hastings L, Boespflug EL, Allendorfer JB, Lamy M, Eliassen JC. Alteration of brain activation patterns in nonallergic rhinitis patients using functional magnetic resonance imaging before and after treatment with intranasal azelastine. Ann Allergy Asthma Immunol. 2011;106(6):527–32. doi:10.1016/j.anai.2011.02.014. Discusses the role of azelastine in modulating the brain response to odorants.
Jiang LH, Gamper N, Beech DJ. Properties and therapeutic potential of transient receptor potential channels with putative roles in adversity: focus on TRPC5, TRPM2 and TRPA1. Curr Drug Targets. 2011;12(5):724–36.
Gao XP, Rubinstein I. Neutral endopeptidase modulates substance P-induced vasodilation in vivo. J Appl Physiol (1985). 1995;78(2):562–8.
Piedimonte G, Hoffman JI, Husseini WK, Bertrand C, Snider RM, Desai MC, et al. Neurogenic vasodilation in the rat nasal mucosa involves neurokinin1 tachykinin receptors. J Pharmacol Exp Ther. 1993;265(1):36–40.
Bhargava D, Bhargava K, Al-Abri A, Al-Bassam W, Al-Abri R. Non allergic rhinitis: prevalence, clinical profile and knowledge gaps in literature. Oman Med J. 2011;26(6):416–20. doi:10.5001/omj.2011.106. Highlights the need to determine the distinct pathogenesis of NAR because of the difficulty in differentiating chronic rhinitis based on symptoms alone.
Bernstein JA. Characterizing rhinitis subtypes. Am J Rhinol Allergy. 2013;27(6):457–60. doi:10.2500/ajra.2013.27.3983. For diagnosis of NAR the cellular, cytokine, genetic, and physiological markers have been less useful. Effective therapeutic response to medications will requ on most appropriate characterization of patient’s chronic rhinitis.
Bernstein JA, Salapatek AM, Lee JS, Nelson V, Wilson D, D’Angelo P, et al. Provocation of nonallergic rhinitis subjects in response to simulated weather conditions using an environmental exposure chamber model. Allergy Asthma Proc. 2012;33(4):333–40. doi:10.2500/aap.2012.33.3579. Study suggest effective investigative techniques and methods to investigate disease mechanisms in NAR and thereby determine and develop novel therapies for NAR.
de Corso E, Battista M, Pandolfini M, Liberati L, Baroni S, Romanello M, et al. Role of inflammation in non-allergic rhinitis. Rhinology. 2014;52(2):142–9. doi:10.4193/Rhin.
Rager JE, Moeller BC, Miller SK, Kracko D, Doyle-Eisele M, Swenberg JA, et al. Formaldehyde-associated changes in microRNAs: tissue and temporal specificity in the rat nose, white blood cells, and bone marrow. Toxicol Sci. 2014;138(1):36–46. doi:10.1093/toxsci/kft267. Study discusses the role of irritants in inducing changes in and deregulation of microRNA leading to alteration in protein expression in the nasal cells of NAR patients.
Suojalehto H, Toskala E, Kilpelainen M, Majuri ML, Mitts C, Lindstrom I, et al. MicroRNA profiles in nasal mucosa of patients with allergic and nonallergic rhinitis and asthma. Int Forum Allergy Rhinol. 2013;3(8):612–20. doi:10.1002/alr.21179. Study determined the roles of specific microRNAs in mediating allergic inflammation in nasal mucosa. But for NAR, the study suggested that mechanisms other than inflammation are pivotal.
Viana F. Chemosensory properties of the trigeminal system. ACS Chem Neurosci. 2011;2(1):38–50. doi:10.1021/cn100102c.
Geppetti P, Patacchini R, Nassini R. Transient receptor potential channels and occupational exposure. Curr Opin Allergy Clin Immunol. 2014;14(2):77–83. doi:10.1097/ACI.0000000000000040. TRP channels on sensory neurons act as sensors of occupational irritant exposures.
Abbott-Banner K, Poll C, Verkuyl JM. Targeting TRP channels in airway disorders. Curr Top Med Chem. 2013;13(3):310–21. The therapeutic potential of TRP channel modulators, the status of these agents in the clinic along with the challenges posed in this rapidly advancing field are also discussed in this review.
Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283(47):32691–703. doi:10.1074/jbc.M803568200.
Patil MJ, Belugin S, Akopian AN. Chronic alteration in phosphatidylinositol 4,5-biphosphate levels regulates capsaicin and mustard oil responses. J Neurosci Res. 2011;89(6):945–54. doi:10.1002/jnr.22597. Depending on coexpression profile of TRPA1 and TRPV1 and cell type the chronic alterations in PIP(2) levels regulate magnitude of response to capsaicin.
Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118(5):1899–910. doi:10.1172/JCI34192.
Grace MS, Belvisi MG. TRPA1 receptors in cough. Pulm Pharmacol Ther. 2011;24(3):286–8. doi:10.1016/j.pupt.2010.11.002.
Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29(8):1568–78. doi:10.1111/j.1460-9568.2009.06702.x.
Guilak F, Leddy HA, Liedtke W. Transient receptor potential vanilloid 4: the sixth sense of the musculoskeletal system? Ann N Y Acad Sci. 2010;1192:404–9. doi:10.1111/j.1749-6632.2010.05389.x.
Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am J Rhinol. 2008;22(1):7–12. doi:10.2500/ajr.2008.22.3125.
Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578(Pt 3):715–33. doi:10.1113/jphysiol.2006.121111.
Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, et al. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi:10.1186/1471-2202-8-49.
Saunders CJ, Christensen M, Finger TE, Tizzano M. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci U S A. 2014;111(16):6075–80. doi:10.1073/pnas.1402251111. Provides insight into the role of solitary chemosensory cells in the airway epithelium that synapse with the trigeminal sensory neurons and therefore may be involved in the pathogenesis of NAR.
Zholos A, Johnson C, Burdyga T, Melanaphy D. TRPM channels in the vasculature. Adv Exp Med Biol. 2011;704:707–29. doi:10.1007/978-94-007-0265-3_37.
Singh U, Bernstein JA. Intranasal capsaicin in management of nonallergic (vasomotor) rhinitis. Prog Drug Res. 2014;68:147–70. Reviews various studies that have determined the efficacy of capsaicin in treating NAR.
Van Gerven L, Alpizar YA, Wouters MM, Hox V, Hauben E, Jorissen M, et al. Capsaicin treatment reduces nasal hyperreactivity and transient receptor potential cation channel subfamily V, receptor 1 (TRPV1) overexpression in patients with idiopathic rhinitis. J Allergy Clin Immunol. 2014;133(5):1332-9, 9 e1-3. doi:10.1016/j.jaci.2013.08.026. Emphasizes the role of TRP channels in development of NAR and thereby the role of TRPV1 agonist, capsaicin in desensitizing the TRPV1 channels, and therefore their suggested use in NAR and inflammatory lower airway disorders.
McGarvey LP, Butler CA, Stokesberry S, Polley L, McQuaid S, Abdullah H, et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J Allergy Clin Immunol. 2014;133:704–12 e4. doi:10.1016/j.jaci.2013.09.016. Determines overexpression of functional TRPV1 channels in the human airway epithelium in the airways of patients with refractory asthma.
Singh U, Bernstein JA, Haar L, Luther K, Jones WK. Azelastine desensitization of transient receptor potential vanilloid 1: a potential mechanism explaining its therapeutic effect in nonallergic rhinitis. Am J Rhinol Allergy. 2014;28(3):215–24. doi:10.2500/ajra.2014.28.4059. Determined the influence of pharmacological agents used in NAR to desensitize TRPV1 channels in sensory neurons and nasal epithelial cells and therefore investigates in-depth mechanism of action of these agents.
Birrell MA, Bonvini SJ, Dubuis E, Maher SA, Wortley MA, Grace MS, et al. Tiotropium modulates transient receptor potential V1 (TRPV1) in airway sensory nerves: a beneficial off-target effect? J Allergy Clin Immunol. 2014;133(3):679–87 e9. doi:10.1016/j.jaci.2013.12.003. Study determined anti-TRPV1 activity of Tiotropium through a mechanism unrelated to its anticholinergic activity.
Musella A, De Chiara V, Rossi S, Cavasinni F, Castelli M, Cantarella C, et al. Transient receptor potential vanilloid 1 channels control acetylcholine/2-arachidonoylglicerol coupling in the striatum. Neuroscience. 2010;167(3):864–71. doi:10.1016/j.neuroscience.2010.02.058.
Dinh QT, Suhling H, Fischer A, Braun A, Welte T. Innervation of the airways in asthma bronchiale and chronic obstructive pulmonary disease (COPD). Pneumologie. 2011;65(5):283–92. doi:10.1055/s-0030-1256123.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63 Suppl 86:8–160. doi:10.1111/j.1398-9995.2007.01620.x.
Eriksson J, Bjerg A, Lotvall J, Wennergren G, Ronmark E, Toren K, et al. Rhinitis phenotypes correlate with different symptom presentation and risk factor patterns of asthma. Respir Med. 2011;105(11):1611–21. doi:10.1016/j.rmed.2011.06.004. Study confirmed a close relationship between nasal disease e.g., allergic rhinitis, chronic rhinitis and chronic rhinosinusitis and asthma.
Delescluse I, Mace H, Adcock JJ. Inhibition of airway hyper-responsiveness by TRPV1 antagonists (SB-705498 and PF-04065463) in the unanaesthetized, ovalbumin-sensitized guinea pig. Br J Pharmacol. 2012;166(6):1822–32. doi:10.1111/j.1476-5381.2012.01891.x. Discusses the role of TRPV1 channels on airway sensory nerves in the pathogenesis of airway hyper-reactivity that hints towards modulation of TRPV1-receptor activity in treating AHR in airway disease.
Kraneveld AD, Kool M, van Houwelingen AH, Roholl P, Solomon A, Postma DS, et al. Elicitation of allergic asthma by immunoglobulin free light chains. Proc Natl Acad Sci U S A. 2005;102(5):1578–83. doi:10.1073/pnas.0406808102.
Bernstein JA, Levin LS, Al-Shuik E, Martin VT. Clinical characteristics of chronic rhinitis patients with high vs low irritant trigger burdens. Ann Allergy Asthma Immunol. 2012;109(3):173–8. doi:10.1016/j.anai.2012.06.013. This article demonstrates that the use of an irritant index scale is useful for phenotyping chronic rhinitis subtypes. Allergic and non-allergic rhinitis patients with a high irritant index scale had increased and more severe symptoms as well as an increase in physician diagnosed asthma.
Ramalho R, Pirraco A, Soares R, Palmares C, Delgado L, Moreira A. Neurogenic inflammation in the airways of elite swimmers. J Sports Med Phys Fitness. 2014;54(2):252–3. Study determined the importance of irritant index questionnaires in reclassification of physician-diagnosed rhinitis patients into different diagnostic categories with unique clinical characteristics.
Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010;7(4):269–77. doi:10.1513/pats.201001-004SM.
Comoglu S, Keles N, Deger K. Inflammatory cell patterns in the nasal mucosa of patients with idiopathic rhinitis. Am J Rhinol Allergy. 2012;26(2):e55–62. doi:10.2500/ajra.2012.26.3725.
Thio M, Blokhuis BR, Nijkamp FP, Redegeld FA. Free immunoglobulin light chains: a novel target in the therapy of inflammatory diseases. Trends Pharmacol Sci. 2008;29(4):170–4. doi:10.1016/j.tips.2008.01.004.
Meng C, Sha J, Li L, An L, Zhu X, Meng X, et al. The expression and significance of immunoglobulin free light chain in the patients with allergic rhinitis and nonallergic rhinitis. Am J Rhinol Allergy. 2014;28(4):302–7. doi:10.2500/ajra.2014.28.4065. Immunoglobulin free light chain may play an important role in inducing local nasal mucosa inflammation especially those in AR and NAR.
Phipps JE, Kestler DP, Foster JS, Kennel SJ, Donnell R, Weiss DT, et al. Inhibition of pathologic immunoglobulin-free light chain production by small interfering RNA molecules. Exp Hematol. 2010;38(11):1006–13. doi:10.1016/j.exphem.2010.07.001.
Lee SW, Kim HJ, Yoo KH, Park YB, Park JY, Jung JY, et al. Long-acting anticholinergic agents in patients with uncontrolled asthma: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2014;18(12):1421–30. doi:10.5588/ijtld.14.0275. Addition of tiotropium may be beneficial for patients with poorly controlled asthma.
Abadoglu O, Berk S. Tiotropium may improve asthma symptoms and lung function in asthmatic patients with irreversible airway obstruction: the real-life data. Clin Respir J. 2014. doi:10.1111/crj.12230. Study demonstrated that addition of tiotropium to standard care may be helpful in poorly controlled asthma despite of the use of ICS/LABA.
Vogelberg C, Engel M, Moroni-Zentgraf P, Leonaviciute-Klimantaviciene M, Sigmund R, Downie J, et al. Tiotropium in asthmatic adolescents symptomatic despite inhaled corticosteroids: a randomised dose-ranging study. Respir Med. 2014;108(9):1268–76. doi:10.1016/j.rmed.2014.06.011. Hints towards the role of LAMA, Tiotropium, as add-on to the current therapeutic approaches to asthma especially if it is refractory to ICS/LABA.
Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A. 2014;111(31):11515–20. doi:10.1073/pnas.1411032111. Highlights the role of sensory neurons in development of asthma.
Zhao L, Wu J, Zhang X, Kuang H, Guo Y, Ma L. The effect of Shenmai injection on the proliferation of rat airway smooth muscle cells in asthma and underlying mechanism. BMC Complement Altern Med. 2013;13:221. doi:10.1186/1472-6882-13-221.
Compliance with Ethics Guidelines
Conflict of Interest
Jonathan Bernstein reports grants and personal fees from Meda and grants from BI. Umesh Singh declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Rhinitis
Rights and permissions
About this article
Cite this article
Bernstein, J.A., Singh, U. Neural Abnormalities in Nonallergic Rhinitis. Curr Allergy Asthma Rep 15, 18 (2015). https://doi.org/10.1007/s11882-015-0511-7
Published:
DOI: https://doi.org/10.1007/s11882-015-0511-7