Abstract
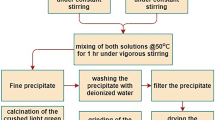
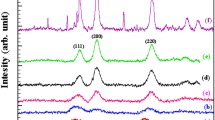
Present work involves synthesis of NiO nanoparticles using chemical homogeneous precipitation (CHP) method as a facile procedure. Ammonia as a complex agent was used in this method. Effects of different types of complexation-precipitation methods on the crystallinity and morphology of nanoparticles were investigated. NiO particles were prepared by direct precipitation method from NiSO4 solution to compare crystallinity and morphology of NiO particles with particles obtained via complexation-precipitation methods. Our major intent was to investigate the effect of complex agent on the crystallization and growth of NiO nanoparticles. Results showed that the best condition for synthesizing spherical NiO shape was using NaOH as decomposing agent, of which the consequence was more uniformity and spherical nanoparticles with a diameter in the range of 40–60 nm. The size of the nickel oxide and nickel hydroxide nanoparticles was estimated by X-ray powder diffraction (XRD) pattern. The chemical structure information of the particles was studied by Fourier transform infrared (FT-IR) spectroscopy. Spherical, elliptical, sheet or flowerlike shapes were detected by field emission scanning electron microscopy (FESEM) analysis. Results showed that by the use of ammonia as complex agent, crystalline state and particles size distribution of NiO nanoparticles improved.
Similar content being viewed by others
References
J. Moghaddam, S. Kolahgar-Azari and S. Karimi, Ind. Eng. Chem. Res., 51, 3224 (2012).
Y. He, K. Vinodgopal, M. Ashokkumar and F. Grieser, Res. Chem. Intermed., 32, 709 (2006).
R.M. Kassab, K. T. Jackson, O. M. El-Kadri and H.M. El-Kaderi, Res. Chem. Intermed., 37, 747 (2011).
D. Adler and J. J. Feinleib, Phys. Rev. B: Condens. Matter, 2, 3112 (1970).
I. Hotovy, J. Huran, L. Spiess, S. Hascik and V. Tehacek, Sens. Actuators, B, 57, 147 (1999).
E. L. Miller and R. E. Rocheleau, J. Electrochem. Soc., 144, 3072 (1997).
Y. P. Wang, J.W. Zhu, X. J. Yang, L.D. Lu and X. Wang, Thermochim. Acta, 437, 106 (2005).
R. C. Makkus, K. Hemmes and J. H.W. D. Wir, J. Electrochem. Soc., 141, 3429 (1994).
M. Ghosh, K. Biswas, A. Sundaresan and C. N. R. Rao, J. Mater. Chem., 16, 106 (2006).
X. Wang, L. J. Ye, P. Hu and F. L. Yuan, Cryst. Growth Des., 7, 2415 (2007).
C.N. Huang, S.Y. Chen and P. Shen, J. Phys. Chem. C, 111, 3322 (2007).
B. Zhao, X. K. Ke and J. H. Bao, J. Phys. Chem. C, 113, 14440 (2009).
M. S. Wu and H. H. Hsieh, Electrochim. Acta, 53, 3427 (2008).
J. R. A. Sietsma, J. D. Meeldijk, J. P. D. Breejen, M. V. Helder, A. J.V. Dillen, P. E. D. Jongh and K. P. D. Jong, Angew. Chem. Int. Ed., 46, 4547 (2007).
L. X. Yang, Y. J. Zhu, H. Tong, Z. H. Liang, L. Li and L. J. Zhang, J. Solid State Chem., 180, 2095 (2007).
C. K. Xu, K. Q. Hong, S. Liu, G.H. Wang and X. N. Zhao, J. Cryst. Growth, 255, 308 (2003).
L. L. Wu, Y. S. Wu, H.Y. Wei, Y. C. Shi and C. X. Hu, Mater. Lett., 58, 2700 (2004).
M.B. Zheng, J.M. Cao, Y. P. Chen, X. J. Ma, S.G. Deng and J. Tao, Chem. Lett., 34, 1174 (2005).
W. Xing, F. Li, Z. F. Yan, H.M. Cheng and G.Q. Lu, Int. J. Nanosci., 3, 321 (2004).
X. M. Liu, X. G. Zhang and S. Y. Fu, Mater. Res. Bull., 41, 620 (2006).
L.Y. Bai, F. L. Yuan, P. Hu, S.K. Yan, X. Wang and S. H. Li, Mater. Lett., 61, 1698 (2007).
X.M. Ni, Y. F. Zhang, D.Y. Tian, H.G. Zheng and X.W. Wang, J. Cryst. Growth, 306, 418 (2007).
A. Al-Hajry, A. Umar, M. Vaseem and M. S. Al-Assiri, Superlattices Microstruct., 44, 216 (2008).
L. P. Zhu, G. H. Liao, Y. Yang, H.M. Zhao and J.G. Wang, Nanoscale Res. Lett., 4, 550 (2009).
H. Z. Wang and Y. T. Qian, Cryst. Res. Technol., 45, 545 (2010).
V. Rehacek, P. Siciliano, S. Capone and L. Spiess, Thin. Solid. Films, 418, 9 (2002).
T.Y. Kim, J.Y. Kim, S. H. Lee, H.W. Shim, S. H. Lee, E. K. Su and K. S. Nahm, Synthetic Met., 144, 61 (2004).
F. Li, H. Chen, Ch. Wang and K. Hu, J. Electroanal. Chem., 531, 53 (2002).
S. A. Needham, G. X. Wang and H. K. Liu, J. Power. Sources, 159, 254 (2006).
M. Gondal, M. Sayeed and Z. Seddigi, J. Hazard. Mater., 155, 83 (2008).
D. B. Kuang, B. X. Lei, Y. P. Pan, X.Y. Yu and Ch.Y. Su, J. Phys. Chem. C, 113, 5508 (2009).
Q. Yang, J. Sha, X. Maa and D. Yang, Mater. Lett., 59, 1967 (2005).
M. Salavati-Niasari, N. Mir and F. Davar, J. Alloy. Compd., 493, 163 (2010).
V.V. Plashnitsa, V. Gupta and N. Miura, Electrochim. Acta, 65, 6941 (2010).
X. Y. Deng and Z. Chen, Mater. Lett., 58, 276 (2004).
P.V. Kamath and G. N. Subbanna, J. Appl. Electrochem., 22, 478 (1992).
Z. Wei, H. Qiao, H. Yang, C. Zhang and X. Yan, J. Alloy. Compd., 479, 855 (2009).
B.D. Cullity, Elements of X-ray diffraction, First Ed., Addison Wesley, Massachusetts (1956).
A. D. Paola, E. García-López, G. MarcÌ and L. Palmisano, J. Hazard. Mater., 211–212, 3 (2012).
Q. S. Song, Y.Y. Li and S. L. I. Chan, J. Appl. Electrochem., 35, 157 (2005).
C. Xu, K. Hong, Sh. Liu, G. Wang and X. Zhao, J. Cryst. Growth, 255, 308 (2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moghaddam, J., Hashemi, E. Fabrication and characterization of NiO nanoparticles by precipitation from aqueous solution. Korean J. Chem. Eng. 31, 503–508 (2014). https://doi.org/10.1007/s11814-013-0233-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-013-0233-2