Abstract
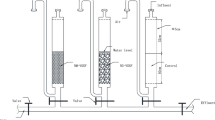
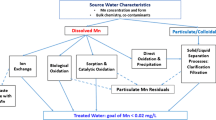
The continuous variations of dissolved oxygen (DO), manganese (Mn), pH, and their effect on manganese removal by different water treatment processes are investigated. The results show that the declined DO concentration and pH value in the bottom of reservoir results in the increasing release of Mn from sediment to source water. Manganese concentration increased from 0.1 to 0.4 mg·L−1 under the condition that DO concentration decreased from 12.0 to 2.0 mg·L−1 in raw water. The different water treatment processes exhibited different efficiency on manganese removal. The processes with recycling of the suspended sludge, low elevation velocity in settling tank and slow filter rate, will benefit the manganese removal. During a high release of manganese in raw water, traditional coagulation-sedimentation and filtration could not completely remove Mn, although granular activated carbon filtration (GAC) had been applied. At that case, preoxidation with chlorine or potassium permanganate (KMnO4) was necessary to address the high manganese concentration.
Similar content being viewed by others
References
Yuce G, Alptekin C. In situ and laboratory treatment tests for lowering of excess manganese and iron in drinking water sourced from river-groundwater interaction. Environmental Earth Science, 2013, 70(6): 2827–2837
Li D, Zeng H P, Zhang J. Review of iron and manganese removal technology in drinking water. Jishui Paishui/Water and Wastewater Engineering, 2011, 37(6): 7–12 (in Chinese)
Martynova M V. Causes of periodic occurrence of high manganese concentrations in Moskva River Reservoirs. Water Resources, 2011, 38(5): 682–683
Zaw M, Chiswell B. Iron and manganese dynamics in lake water. Water Research, 1999, 33(8): 1900–1910
Crimi M, Ko S. Control of manganese dioxide particles resulting from in situ chemical oxidation using permanganate. Chemosphere, 2009, 74(6): 847–853
Kim J, Jung S. Soluble manganese removal by porous media filtration. Environmental Technology, 2008, 29(12): 1265–1273
Betancourt C, Jorge F, Suárez R, Beutel M, Gebremariam S. Manganese sources and cycling in a tropical eutrophic water supply reservoir, Paso Bonito Reservoir, Cuba. Lake and Reservoir Management, 2010, 26(3): 217–226
Burger M S, Krentz C A, Mercer S S, Gagnon G A. Manganese removal and occurrence of manganese oxidizing bacteria in fullscale biofilters. Journal of Water Supply: Research & Technology-Aqua, 2008, 57(5): 351–359
Tiwari D, Yu M R, Kim M N, Lee S M, Kwon O H, Choi K M, Lim G J, Yang J K. Potential application of manganese coated sand in the removal of Mn(II) from aqueous solutions. Water Science and Technology, 2007, 56(7): 153–160
Long B W, Hulsey R A, Hoehn R C. Complementary uses of chlorine dioxide and ozone for drinking water treatment. Ozone Science and Engineering, 1999, 21(5): 465–476
Hu C Z, Liu H J, Qu J H, Wang D S, Rut J. Coagulation behavior of aluminum salts in eutrophic water: significance of Al13 species and pH control. Environmental Science & Technology, 2006, 40(1): 325–331
Jarvis P, Jefferson B, Parsons S A. Breakage, regrowth, and fractal nature of natural organic matter flocs. Environmental Science & Technology, 2005, 39(7): 2307–2314
Liang L, Singer P C. Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environmental Science & Technology, 2003, 37(13): 2920–2928
Cerrato J M, Falkinham J O Dietrich A M, KnockeW R, McKinney C W, Pruden A. Manganese-oxidizing and -reducing microorganisms isolated from biofilms in chlorinated drinking water systems. Water Research, 2010, 44(13): 3935–3945
Gantzer P A, Bryant L D, Little J C. Controlling soluble iron and manganese in a water-supply reservoir using hypolimnetic oxygenation. Water Research, 2009, 43(5): 1285–1294
Burger M S, Mercer S S, Shupe G D, Gagnon G A. Manganese removal during bench-scale biofiltration. Water Research, 2008, 42(19): 4733–4742
Tekerlekopoulou A G, Vasiliadou I A, Vayenas D V. Biological manganese removal from potable water using trickling filters. Biochemical Engineering Journal, 2008, 38(3): 292–301
Dong D, Li Y, Hua X Y. Investigation of Fe, Mn oxides and organic material in surface coatings and Pb, Cd adsorption to surface coatings developed in different natural waters. Microchemical Journal, 2001, 70(1): 25–33
Fernández S, Villanueva U, de Diego A, Arana G, Madariaga J M. Monitoring trace elements (Al, As, Cr, Cu, Fe, Mn, Ni and Zn) in deep and surface waters of the estuary of the Nerbioi-Ibaizabal River (Bay of Biscay, Basque Country). Journal of Marine Systems, 2008, 72(1–4): 332–341
Gouzinis A, Kosmidis N, Vayenas D V, Lyberatos G. Removal of Mn and simultaneous removal of NH3, Fe and Mn from potable water using a trickling filter. Water Research, 1998, 32(8): 2442–2450
Wang L. Study on water quality transformation in Miyun Reservoir. China Water & Wastewater, 2006, 22(13): 45–48 (in Chinese)
Matilainen A, Lindqvist N, Tuhkanen T. Comparison of the efficiency of aluminium and ferric sulphate in the removal of natural organic matter during drinking water treatment process. Environmental Technology, 2005, 26: 867–875
Wu X L, Tan X L, Yang S T, Wen T, Guo H L, Wang X K, Xu A W. Coexistence of adsorption and coagulation processes of both arsenate and NOM from contaminated groundwater by nanocrystallined Mg/Al layered double hydroxides. Water Research, 2013, 47: 4159–4168
National Standards of the People’s Republic of China. GB/T 5750-2006, Standard Examination Methods for Drinking Water-Metal Parameters. Beijing: National Standards of the People’s Republic of China, 2006
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Xiao, F., Liu, Y. et al. Occurance and control of manganese in a large scale water treatment plant. Front. Environ. Sci. Eng. 9, 66–72 (2015). https://doi.org/10.1007/s11783-014-0637-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-014-0637-1