Abstract
Idiopathic pulmonary fibrosis (IPF) is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause. IPF is associated with an increased risk of lung cancer, and lung cancer patients with IPF undergoing pulmonary resection for non-small cell lung cancer have increased postoperative morbidity and mortality. Especially, postoperative acute exacerbation of IPF (AEIPF) causes fatal status and long-term outcomes are worse than for patients without IPF, although certain subgroups have a good long-term outcome. A comprehensive review of the current literature pertaining to AEIPF and the late phase outcome after the context of a surgical intervention was performed.
Similar content being viewed by others
Introduction
Pulmonary fibrosis is associated with an increased risk of lung cancer, with a relative risk of 7.0–14.0 (compared to the general population) [1]. Thoracic surgeons often face difficulties treating lung cancer patients with idiopathic pulmonary fibrosis (IPF) because of poor prognosis of IPF itself and the high morbidity/mortality rate after pulmonary resection [2–4]. The Japanese Association for Thoracic Surgery has conducted a survey of lung cancer surgery, published in the annual report for 2008 [5]. In 1,036 of 27,881 lung cancer patients, interstitial pneumonia (IP: including the other IP excluding IPF) was diagnosed as an associated disease. Sixty-eight patients died of IP within 30 days post operation and this IP, probably with acute exacerbation (AE), was the major cause of the post-operative deaths. In the perioperative morbidity, AE of IP is one of the most common factors of fatality in IP patients.
This article aims to review outcomes of lung resection for non-small cell cancer patients concomitant with IPF. It focuses on the current status and future outlook.
Epidemiology and natural history of IPF
Thoracic surgeons need to know the epidemiology and natural history of IPF in order to treat IPF patients. There are few large-scale studies of the incidence or prevalence of IPF. In a population-based study, the incidence of IPF for men and women were estimated as 10.7 and 7.4 per 100,000 cases per year, respectively [6]. Another study from the United States estimated the incidence of IPF to be between 6.8 and 16.3 per 100,000 persons using a large database of healthcare claims in a health plan [7].
The natural history of IPF has been described as a progressive decline in subjective and objective pulmonary function until eventual death from respiratory failure or complicating co-morbidity [8]. Several retrospective studies suggest that a median survival time is from 2 to 3 years from the time of diagnosis, and 5-year survival rate is less than 30 % [9–11]. There appear to be several possible natural histories for patients with IPF, but the natural history of these patients is unpredictable at the time of the diagnosis [12]. On the other hand, du Bois et al. [13] reported that a clinical model comprising only four predictors (age, respiratory hospitalization, percent predicted FVC, and 24-week change in FVC), and the corresponding risk scoring system produced estimates of 1-year mortality risk consistent with observed data (9.9 vs. 9.7 %; 95 % confidence interval, 0.71–0.79). It should be noted that the majority of patients demonstrate a slow, gradual progression over many year; that some patients remain stable and that still others have an accelerated decline and some patients may experience episodes of acute respiratory worsening [14].
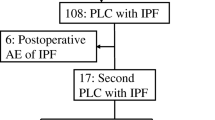
The recent studies provide information regarding the incidence and mortality of the AEIPF. A randomized, controlled trial of anticoagulant therapy in IPF included rehospitalization (which included AE) as a secondary endpoint [15]. Of 56 patients followed for approximately 3 years, 32 (57 %) were rehospitalized for AE and 53 % of those patients died. A retrospective review of an observational cohort of 147 patients with IPF identified 11 who met criteria for AE [16]. The 2-year incidence of AE was reported at 9.6 %, and mortality at 78 %.
Acute exacerbation of idiopathic pulmonary fibrosis (AEIPF)
Diagnostic criteria
Recent observations have suggested that acute respiratory worsening occurs in a small minority of patients with IPF annually (approximately 5–10 %) [17, 18].
The AEIPF is characterized by diffuse and rapid alveolar damage superimposed on a background of IPF, which most likely occurs as a result of a massive lung injury due to some unknown etiologic agent. The definition of AEIPF was first described by Yoshimura et al. [19]. The characteristics include (1) intensified dyspnea, (2) an increase in the interstitial shadow on the chest radiograph, (3) an increase in fine crackles on auscultation, (4) an elevation of serum lactate dehydrogenase, and (5) a decrease in arterial oxygen tension of more than 10 mmHg under similar conditions. After then, some diagnostic criteria have been described in Table 1 [17, 20–22].
Etiology
There are two competing hypotheses regarding the pathogenesis of AEIPF. One hypothesis posits that AEIPF represent a distinct but intrinsic manifestation of the underlying disease process representing accelerated or decompensated IPF. The host’s susceptibility to fibrosis alone predisposes patients to their AEs. The second hypothesis holds that AEs are the result of a clinically occult and often unrecognized extrinsic trigger such as a viral infection or silent aspiration [23].
The role of circulating fibrocytes may provide a possible mechanism for an AE in the postoperative period. Circulating fibrocytes (CF) were first described by Bucala et al. [24] as circulating cells that concentrate in wound tissue and contribute to wound repair. Moeller et al. [25] demonstrated that the levels of CFs increase during an AEIPF and then return to their baseline levels, as in the stable state of IPF in patients who survive the episode of AE. Interestingly, the levels of CFs in their patients with ALI/ARDS were comparable to those of healthy volunteers and stable IPF patients and at statistically significantly lower levels than those of patients in an AEIPF. This finding indicates that measurement of CFs in IPF patients may correlate with fibrogenic activity and may be an indication of disease progression.
Konishi et al. [26] analyzed global gene expression patterns in the lungs of non-surgical patients undergoing AEIPF and compared them with stable IPF and control lungs. According to this research, AEIPF is characterized by enhanced epithelial injury and proliferation, as reflected by increases in CCNA2 and α-defensins and an apoptosis of epithelium. The concomitant increase in α-defensins in the peripheral blood and lungs may suggest their use as biomarkers for this disorder. These biomarkers and genes may play a significant role for preoperative prediction of the occurrence of AEIPF.
Some unknown or potential etiologic agents of AEIPF must be induced by pulmonary resection or some factors related to pulmonary resection, such as selective lung ventilation and mechanical retraction of the ipsilateral lung. Shortening one lung ventilation and avoiding pulmonary retraction may inhibit the occurrence of AEIPF, resulting from oxygen-derived free radicals [4].
Several investigations have reported the AEIPF following surgery for lung cancer in preoperative IPF patients, and have described several risk predictors of AEIPF.
Furthermore, some researchers focus on intraoperative ventilation with respect to postoperative AEIPF. The injury could be mediated by a higher oxygen concentration. Another possible mechanism is that the insult could be mechanical rather than chemical, secondary to higher airway pressures or higher tidal volumes required on the ventilated side during one lung ventilation. Importantly, these insults occur in the setting of severely diseased lungs with reduced compliance, suggesting that hyperoxia, barotrauma, and volutrauma may play roles in the diffuse alveolar damage [27]. Furthermore, Licker et al. [28] reported that early onset acute lung injury after pulmonary resections in non-small cell lung cancer (NSCLC) patients associated with preoperative alcohol consumption, pneumonectomy, high intraoperative pressure index, and excessive fluid intake over the first 24 h.
The incidence (0–32 %), predictive risk and mortality (0–22.9 %) of postoperative AEIPF are summarized in Table 2.
Prophylactic therapy
The differences of surgical approaches, such as conventional thoracotomy, muscle sparing thoracotomy, and video-assisted thoracoscopic surgery do not have an effect on the occurrence of postoperative AEIPF [29]. There is one report that the intraoperative fluid balance is a prognostic factor for postoperative AEIPF [P = 0.026, odds ratio (OR) = 1.312] [30]. On the other hand, with respect to water balance during surgery, Okamoto et al. [3] reported no significant difference between patients who did and did not develop postoperative AEIPF. We think that the intraoperative water balance is the secondary deterioration cause of AEIPF, but not the primary cause.
Unfortunately, no drug has been developed to decrease the incidence of AEIPF. Few studies reported that steroid, pirfenidone [18], and anticoagulants [15] reduce the AEIPF occurrence in IPF patients. However, regarding postoperative AEIPF, so far, no research has presented a drug that can prevent or decrease the occurrence of postoperative AEIPF. Thus, thoracic surgeons use some drugs reported to reduce the incidence of AEIPF or to slow the deterioration of IPF, such as macrolides [31, 32]. N-acetylcysteine [33, 34], proteinase inhibitor [35, 36] and pirfenidone [37, 38]. The effect of these drugs on decreasing the incidence of postoperative AEIPF remains unclear; therefore, multi-institutional randomized controlled studies should be planned in order to determine their effects [39].
Therapy for AEIPF
Once the diagnosis of an AEIPF has been made, the therapeutic options still remain debated. There is little evidence that currently accepted treatments are effective in AEIPF, and further studies are needed to clarify the pathogenesis and contribute to the prevention of AEIPF. Commonly used options are steroids [40], immunosuppressants (such as Cyclosporine A [41, 42] and cyclophosphamide [43]), anticoagulation [15] and Sivelestat sodium hydrate (a specific neutrophils elastase inhibitor which may protect endothelial cells against neutrophil-mediated injury by inactivating the extracellular elastase secreted by neutrophils, and also by acting directly on neutrophils to suppress the production and secretion of activated elastase) [44]. In truth, however, there are no data from controlled trials to support the efficacy of these treatments.
There are some reports on the effect of hemoperfusion [45, 46] for AEIPF treatment. In four of six AEIPF patients, a polymyxin B-immobilized fiber column (PMX) hemoperfusion treatment improved alveolar-arterial difference of oxygen, serum KL-6, and lactate dehydrogenase levels. These four patients were successfully weaned from mechanical ventilation and survived more than 30 days after the initial PMX treatment. The limitation of this study is the small number of the patients, and multicenter trials are needed.
Many thoracic surgeons may consider ventilator support at a postoperative necessity of AEIPF. However, it is important to note that patients with IPF admitted to the intensive care unit with acute respiratory failure needing mechanical ventilation have a very poor prognosis [47]. In the period 1980–2000, many studies highlighted the inadequacy of mechanical ventilation in acute respiratory failure in IPF. Pooled data showed an aggregated mortality of 87 % (118 out of 135), and short-term mortality (within 3 months of hospital discharge) of 94 % [48, 49].
Acute phase morbidity and mortality
It remains controversial if morbidity excluding AEIPF after lung resection for lung cancer is impacted by comorbidity of IPF. Postoperative morbidity rates did not relate to the comorbidity of IPF. However, patients with IPF had adverse pulmonary events more frequently than those without IPF (26 vs. 9.1 %, respectively, P < 0.01), including pneumonia (15 %), prolonged air leakage (13 %), bronchopleural fistula (6 %), and empyema (4 %). Patients with IPF showed a higher mortality than those without IPF (8 vs. 0.8 %, respectively, P < 0.01) [50]. Postoperative morbidity rates were 40.7 % for patients with IPF versus 18.8 % for patients without IPF (P = 0.007) and pulmonary complications were 35.7 and 12.7 % (P = 0.001), respectively. Furthermore, patients with IPF showed a 3.6 % rate of 90-day mortality versus 0.3 % for those without IPF (P = 0.028) [51]. These results show that not only AEIPF but also other respiratory complications increase after lung resection.
Long-term outcome
Recurrence
It remains unclear whether lung cancer patients with IPF have an increased recurrence rate. It is very difficult to distinguish recurrence of primary cancer from heterochronic double cancer. In previous reports, the rate has not been reported. However, as will be mentioned later, the major cause of death in these patients after lung resection is cancer related. The cancer recurrence rate might be higher in patients with IPF than those without.
Survival and cause of death during late phase
Few investigators have reported on the long-term outcome after lung resection for NSCLC patients with IPF. Saito et al. [51] reported that the 5-year survival rates were 54.2 % in pathologic stage IA lung cancer patients with IPF and 88.3 % in those without IPF (P < 0.0001). In this study, multivariate analysis showed that only IPF was a significant prognostic factor for survival (P = 0.007). Our previous reports described that the postoperative 5-year survival for pathologic stage I lung cancer was 61.6 % for patients with IPF and 83.0 % for patients without, and disease-free survival at year 5 was 56.0 and 83.1 %, respectively [4]. Furthermore, the cause of death during late phase was 50 % from lung cancer and 31.3 % from IPF related respiratory failure.
These results and the other report data are summarized in Table 3. It should be noted that lung cancer patients with IPF have died of lung cancer rather than respiratory failure due to deterioration of IPF. Further study on the outcome of treatments of primary lung cancer combined with IPF with a large number of patients is expected.
Surgical strategy
A definitive estimation of preoperative N (node) status is very important because the discrepancy between the clinical and the pathological N status is sometimes observed due to swelling of the lymph nodes, which is related to persistent inflammation in the lung parenchyma caused by IPF. PET/CT offers significantly increased accuracy versus CT in mediastinal nodal staging in patients with NSCLC and IPF compared with patients with NSCLC but without IPF, mainly because of improved specificity. However, the incremental accuracy of PET/CT, which was 33 % for patients with IPF and 14 % for patients without IPF [52]. Therefore, definite preoperative staging by endobronchial ultrasonography-transbronchial needle aspiration (EBUS-TBNA) or mediastinoscopy is recommended because N2 patients have an extremely poor prognosis even after curative resection.
Some researchers have reported that a limited resection is acceptable if the resection can be achieved with an adequate margin [53]. Surely, a limited resection might decrease the occurrence of AEIPF. On the other hand, a limited resection might increase the recurrence rate and decrease survival rate. Surgical strategy on a volume of lung resection remains controversial when we consider the risk of AEIPF and late term outcome. We think that a lobectomy should be done if the patients can tolerate it. Pneumonectomy should be avoided for the same reason because it is associated with major morbidity.
Strategy for recurrence or second development of lung cancer
In NSCLC patients with IPF, it was very difficult to undergo reresection because the first lung resection reduces their pulmonary functioning. The reduction of postoperative pulmonary functioning is more than a preoperative predictive value and postoperative value of those without IPF [4]. On the other hand, chemotherapy and/or radiotherapy could possibly contribute to the development of pulmonary fibrosis. The incidence of AE related to anticancer treatment was 22.7 %; when the incidence was examined separately for patients receiving chemotherapy or the best supportive care, the incidence was 20.0 and 31.3 %, respectively. Combination regimens of carboplatin + paclitaxel or a platinum agent + etoposide significantly reduced the incidence of AE in comparison with other regimens (0 vs. 18 %) [54]. When using these regimens, one should take extremely good care of the patients with IPF.
Future outlook
A retrospective multi-institutional survey about NSCLC patients with IPF who underwent lung resection was planned and performed by the educational committee of the Japanese association of chest surgery. Acute exacerbation of interstitial pneumonia (AEIP) developed in about 10 % of these patients and 40 % of AEIP developing patients died. This study is expected to reveal the predictive risk factors of AEIP after lung resection. The detailed results will be reported in near future. Our surgical strategy for NSCLC patients with IPF might be improved by these data.
We present several questions that should be tested in future research studies. First, what is effective prophylactic therapy and treatment of AEIPF after lung resection? Second, which is a proper ventilation treatment for postoperative AEIPF patients, intubation and mechanical ventilation or non-invasive ventilation? Third, what is the proper surgical option and inclusion criteria for NSCLC patients with IPF. To answer these questions, systematic and controlled multi-institutional study is needed. The development of multi-institutional registries to prospectively collect clinical data and biological samples from these patients would allow investigators to gain sufficient numbers of subjects and to successfully study this condition.
References
Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population based cohort study. Am J Respir Crit Care Med. 2000;161(1):5–8.
Ozawa Y, Suda T, Naito T, Enomoto N, Hashimoto D, Fujisawa T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology. 2009;14(5):723–8.
Okamoto T, Gotoh M, Masuya D, Nakashima T, Liu D, Kameyama K, et al. Clinical analysis of interstitial pneumonia after surgery for lung cancer. Jpn J Thorac Cardiovasc Surg. 2004;52(7):323–9.
Watanabe A, Higami T, Ohori S, Koyanagi T, Nakashima S, Mawatari T. Is lung cancer resection indicated in patients with idiopathic pulmonary fibrosis? J Thorac Cardiovasc Surg. 2008;136(5):1357–63.
Sakata R, Fujii Y, Kuwano H. Thoracic and cardiovascular surgery in Japan during 2008: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2010;58(7):356–83.
Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150(4):967–72.
Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–6.
Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–25.
Flaherty KR, Toews GB, Travis WD, Colby TV, Kazerooni EA, Gross BH, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J. 2002;19:275–83.
Rudd RM, Prescott RJ, Chalmers JC, Johnston IDA. Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society. British Thoracic Society Study on cryptogenic fibrosing alveolitis: response to treatment and survival. Thorax. 2007;62:62–6.
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An Official ATS/ERS/JRS/ALAT Statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824.
Raghu G. Idiopathic pulmonary fibrosis: a rational clinical approach. Chest. 1987;92(1):148–54.
du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(4):459–66.
Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, Cisneros J, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS One. 2007;2:e482.
Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, Watanabe M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128(3):1475–82.
Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. 2006;27(1):143–50.
Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, et al.; Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176(7):636–43.
Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–7.
Yoshimura K, Nakatani T, Nakamori Y. Acute exacerbation in idiopathic interstitial pneumonia. Nihon Kokyuki Gakkai Zasshi. 1984;22(11):1012–20. (Japanese).
Akira M, Hamada H, Sakatani M, Kobayashi C, Nishioka M, Yamamoto S. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. Am J Roentgenol. 1997;168(1):79–83.
Swigris J, Brown K. Acute interstitial pneumonia and acute exacerbations of idiopathic pulmonary fibrosis. Semin Respir Crit Care Med. 2006;27(6):659–67.
Hyzy R, Huang S, Myers J, Flaherty K, Martinez F. Acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2007;132(5):1652–8.
Huie TJ, Moss M, Frankel SK. What can biomarkers tell us about the pathogenesis of acute exacerbations of idiopathic pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol. 2010;299(1):L1–2.
Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81.
Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(7):588–94.
Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180(2):167–75.
Ghatol A, Ruhl AP, Danoff SK. Exacerbations in idiopathic pulmonary fibrosis triggered by pulmonary and nonpulmonary surgery: a case series and comprehensive review of the literature. Lung. 2012;190(4):373–80.
Licker M, de Perrot M, Spiliopoulos A, Robert J, Diaper J, Chevalley C, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97(6):1558–65.
Koizumi K, Hirata T, Hirai K, Mikami I, Okada D, Yamagishi S, et al. Surgical treatment of lung cancer combined with interstitial pneumonia: the effect of surgical approach on postoperative acute exacerbation. Ann Thorac Cardiovasc Surg 2004;10(6):340–6.
Mizuno Y, Iwata H, Shirahashi K, Takamochi K, Oh S, Suzuki K, et al. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg. 2012;41(6):e161–5.
Azuma A, Furuta T, Enomoto T, Hashimoto Y, Uematsu K, Nukariya N, et al. Preventive effect of erythromycin on experimental bleomycin-induced acute lung injury in rats. Thorax. 1998;53(3):186–9.
Li Y, Azuma A, Takahashi S, Usuki J, Matsuda K, Aoyama A, et al. Fourteen-membered ring macrolides inhibit vascular cell adhesion molecule 1 messenger RNA induction and leukocyte migration: role in preventing lung injury and fibrosis in bleomycin-challenged mice. Chest. 2002;122(6):2137–45.
Radomska-Leśniewska DM, Skopińska-Rózewska E, Jankowska-Steifer E, Sobiecka M, Sadowska AM, Hevelke A, et al. N-acetylcysteine inhibits IL-8 and MMP-9 release and ICAM-1 expression by bronchoalveolar cells from interstitial lung disease patients. Pharmacol Rep. 2010;62(1):131–8.
Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al.; IFIGENIA Study Group. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005;353(21):2229–42.
Taooka Y, Maeda A, Hiyama K, Ishioka S, Yamakido M. Effects of neutrophil elastase inhibitor on bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 1997;156(1):260–5.
Nakamura M, Ogura T, Miyazawa N, Tagawa A, Kozawa S, Watanuki Y, et al. Outcome of patients with acute exacerbation of idiopathic interstitial fibrosis (IPF) treated with sivelestat and the prognostic value of serum KL-6 and surfactant protein D. Nihon Kokyuki Gakkai Zasshi 2007;45(6):455–459 (Japanese).
Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, openlabel phase II study. Am J Respir Crit Care Med. 1999;159(4):1061–9.
Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone Clinical Study Group in Japan. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–9.
Watanabe A, Kawaharada N, Higami T. Postoperative acute exacerbation of IPF after lung resection for primary lung cancer. Pulm Med. 2011;2011:960316.
Panos RJ, Mortenson RL, Niccoli SA, King TE Jr. Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med. 1990;88(4):396–404.
Inase N, Sawada M, Ohtani Y, Miyake S, Isogai S, Sakashita H, et al. Cyclosporin A followed by the treatment of acute exacerbation of idiopathic pulmonary fibrosis with corticosteroid. Intern Med. 2003;42(7):565–70.
Sakamoto S, Homma S, Miyamoto A, Kurosaki A, Fujii T, Yoshimura K. Cyclosporin A in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2010;49(2):109–15.
Ambrosini V, Cancellieri A, Chilosi M, Zompatori M, Trisolini R, Saragoni L, et al. Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J. 2003;22(5):821–6.
Nakatani K, Takeshita S, Tsujimoto H, Kawamura Y, Sekine I. Inhibitory effect of serine protease inhibitors on neutrophil-mediated endothelial cell injury. J Leukoc Biol. 2001;69(2):241–7.
Seo Y, Abe S, Kurahara M, Okada D, Saito Y, Usuki J, et al. Beneficial effect of polymyxin B-immobilized fiber column (PMX) hemoperfusion treatment on acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2006;45(18):1033–8.
Enomoto N, Suda T, Uto T, Kato M, Kaida Y, Ozawa Y, et al. Possible therapeutic effect of direct haemoperfusion with a polymyxin B immobilized fibre column (PMX-DHP) on pulmonary oxygenation in acute exacerbations of interstitial pneumonia. Respirology. 2008;13(3):452–60.
Blivet S, Philit F, Sab JM, Langevin B, Paret M, Guérin C, et al. Outcome of patients with idiopathic pulmonary fibrosis admitted to the ICU for respiratory failure. Chest. 2001;120(1):209–12.
Al-Hameed FM, Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J. 2004;11(2):117–22.
Stern JB, Mal H, Groussard O, Brugière O, Marceau A, Jebrak G, et al. Prognosis of patients with advanced idiopathic pulmonary fibrosis requiring mechanical ventilation for acute respiratory failure. Chest. 2001;120(1):213–9.
Kawasaki H, Nagai K, Yoshida J, Nishimura M, Nishiwaki Y. Postoperative morbidity, mortality, and survival in lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol. 2002;81(1):33–7.
Saito Y, Kawai Y, Takahashi N, Ikeya T, Murai K, Kawabata Y, et al. Survival after surgery for pathologic stage IA non-small cell lung cancer associated with idiopathic pulmonary fibrosis. Ann Thorac Surg. 2011;92(5):1812–7.
Jeon TY, Lee KS, Yi CA, Chung MP, Kwon OJ, Kim BT, et al. Incremental value of PET/CT over CT for mediastinal nodal staging of non-small cell lung cancer: comparison between patients with and without idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 2010;195(2):370–6.
Fujimoto T, Okazaki T, Matsukura T, Hanawa T, Yamashita N, Nishimura K, et al. Operation for lung cancer in patients with idiopathic pulmonary fibrosis: surgical contraindication? Ann Thorac Surg. 2003;76(5):1674–9.
Minegishi Y, Takenaka K, Mizutani H, Sudoh J, Noro R, Okano T, et al. Exacerbation of idiopathic interstitial pneumonias associated with lung cancer therapy. Intern Med. 2009;48(9):665–72.
Kumar P, Goldstraw P, Yamada K, Nicholson AG, Wells AU, Hansell DM, et al. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg. 2003;125(6):1321–7.
Chiyo M, Sekine Y, Iwata T, Tatsumi K, Yasufuku K, Iyoda A, et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: analyses of short-term and long-term outcomes. J Thorac Cardiovasc Surg. 2003;126(4):1141–6.
Kushibe K, Kawaguchi T, Takahama M, Kimura M, Tojo T, Taniguchi S. Operative indications for lung cancer with idiopathic pulmonary fibrosis. Thorac Cardiovasc Surg. 2007;55(8):505–8.
Shintani Y, Ohta M, Iwasaki T, Ikeda N, Tomita E, Kawahara K, et al. Predictive factors for postoperative acute exacerbation of interstitial pneumonia combined with lung cancer. Gen Thorac Cardiovasc Surg. 2010;58(4):182–5.
Yano M, Sasaki H, Moriyama S, Hikosaka Y, Yokota K, Kobayashi S, et al. Post-operative acute exacerbation of pulmonary fibrosis in lung cancer patients undergoing lung resection. Interact Cardiovasc Thorac Surg. 2012;14(2):146–50.
Suzuki H, Sekine Y, Yoshida S, Suzuki M, Shibuya K, Yonemori Y, et al. Risk of acute exacerbation of interstitial pneumonia after pulmonary resection for lung cancer in patients with idiopathic pulmonary fibrosis based on preoperative high-resolution computed tomography. Surg Today. 2011;41(7):914–21.
Author information
Authors and Affiliations
Corresponding author
Additional information
The review was submitted at the invitation of the editorial committee.
Rights and permissions
About this article
Cite this article
Watanabe, A., Miyajima, M., Mishina, T. et al. Surgical treatment for primary lung cancer combined with idiopathic pulmonary fibrosis. Gen Thorac Cardiovasc Surg 61, 254–261 (2013). https://doi.org/10.1007/s11748-012-0180-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-012-0180-6