Abstract
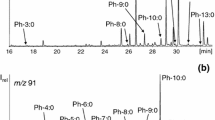
Milk fat is known to contain one of the highest number of fatty acids of all edible oils. Some of these fatty acids are known to be valuable (e.g. conjugated linoleic acids, furan fatty acid) and other as undesirable (e.g. saturated and some trans-fatty acids) food ingredients. However, a comprehensive picture on the presence of many trace fatty acids has not been achieved. For this reason we have developed an analysis scheme based on the conversion of the fatty acids into methyl esters. The fatty acid methyl esters were then fractionated by urea complexation. Both the filtrate of the urea complexation (~4 % of the sample weight) and the original sample were fractionated by high-speed counter-current chromatography (HSCCC). The resulting fractions were analyzed by GC/MS analysis. With this method 430 fatty acids were detected in one single butter sample. More than 230 fatty acids had two or more double bonds. In addition to the widely known spectrum of fatty acids we also detected a range of cyclohexyl fatty acids (five homologues) and methyl-branched fatty acids (including short chain and even-numbered anteiso-fatty acids), conjugated tetradecadienoic acids along with the novel ω-oxo-fatty acids (seven homologues). The reported relative retention time on the polar column may serve as a data base for the screening of other samples for this profusion of fatty acids.








Similar content being viewed by others
Abbreviations
- 9D5/F-VII:
-
9-(3,4-Dimethyl-5-pentylfuran-2-yl)-nonanoic acid
- 9M5/F-III:
-
9-(3-Methyl-5-pentylfuran-2-yl)-nonanoic acid
- 11D5/F-VIII:
-
11-(3,4-Dimethyl-5-pentylfuran-2-yl)-undecanoic acid
- 11M5/F-IV:
-
11-(3-Methyl-5-pentylfuran-2-yl)-undecanoic acid
- a11:0:
-
8-Methyl-decanoic acid
- a15:0:
-
12-Methyl-tetradecanoic acid
- a17:0:
-
14-Methyl-hexadecanoic acid
- CLA:
-
Conjugated linoleic acid
- CTA:
-
Conjugated tetradecadienoic acid
- FAME:
-
Fatty acid methyl ester(s)
- GC:
-
Gas chromatography
- GC/MS:
-
Gas chromatography with mass spectrometry
- HSCCC:
-
High-speed counter-current chromatography
- i15:0:
-
13-Methyl-tetradecanoic acid
- i17:0:
-
15-Methyl-hexadecanoic acid
- KU/L :
-
Partitioning coefficient (upper to lower phase)
- MBFA:
-
Methyl branched fatty acid(s)
- PUFA:
-
Polyunsaturated fatty acid(s)
- SIM:
-
Selected ion monitoring mode
References
Hu FB, Manson JE, Willett WC (2001) Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr 20:5–19
Shorland FB, Weenink RO, Johns AT, McDonalds IRC (1957) The effect of sheep-rumen contents on unsaturated fatty acids. Biochem J 67:328–333
Polan CE, McNeill JJ, Tove SB (1964) Biohydrogenation of unsaturated fatty acids by rumen bacteria. J Bacteriol 88:1056–1064
Chilliard Y, Ferlay A, Mansbridge RM, Doreau M (2000) Ruminant milk fat plasticity: nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. Ann Zootech 49:181–205
Lindmark Månsson H (2008) Fatty acids in bovine milk fat. Food Nutr Res 52. doi:10.3402/fnr.v52i0.1821
Jensen RG (2002) The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci 85:295–350
Willett WC, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Rosner BA, Sampson LA, Hennekens CH (1993) Intake of trans fatty acids and risk of coronary heart disease among women. Lancet 341:581–585
Wahle KWJ, Heys SD, Rotondo D (2004) Conjugated linoleic acids: are they beneficial or detrimental to health? Prog Lipid Res 43:553–587
Parodi PW (2009) Milk fat nutrition. In: Tamime AY (ed) Dairy fats and related products. Blackwell, Oxford (ISBN: 978-1-405-15090-3)
Spitteler G (2005) Furan fatty acids: occurrence, synthesis, and reactions. Are furan fatty acids responsible for the cardioprotective effects of a fish diet? Lipids 40:711–755
Bengen MF (1940) Deutsche Patentanmeldung O.Z. 12438
Bengen F, Schlenk W (1949) Über neuartige Additionsverbindungen des Harnstoffs. Experientia 5:200
Täufel K, Müller G, Franzke CL (1958) Über die Adduktbildung langkettiger Fettsäuren mit Harnstoff. Nahrung 2:255–267
Ito Y (2005) Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J Chromatogr A 1065:145–168
Degenhardt A, Engelhardt UH, Lakenbrink C, Winterhalter P (2000) Preparative separation of polyphenols from tea by high-speed countercurrent chromatography. J Agric Food Chem 48:3425–3430
Montilla EC, Hillebrand S, Butschbach D, Baldermann S, Watanabe N, Winterhalter P (2010) Preparative isolation of anthocyanins from Japanese purple sweet potato (Ipomoea batatas L.) varieties by high-speed countercurrent chromatography. J Agric Food Chem 58:9899–9904
Koehler N, Wray V, Winterhalter P (2008) Preparative isolation of procyanidins from grape seed extracts by high-speed counter-current chromatography. J Chromatogr A 1177:114–125
Zhou Y, Chen F, Li Z (2002) Preparative separation of beta-sitosterol by high speed countercurrent chromatography. J Liq Chromatogr Relat Technol 25:1693–1701
Schröder M, Vetter W (2011) High-speed counter-current chromatographic separation of phytosterols. Anal Bioanal Chem 400:3615–3623
Schröder M, Vetter W (2012) Investigation of unsaponifiable matter of plant oils and isolation of eight phytosterols by means of high-speed counter-current chromatography. J Chromatogr A 1237:96–105
Huang L, Cao X, Xu H, Chen G (2011) Separation and purification of ergosterol and stigmasterol in Anoectochilus roxburghii (wall) lindl by high-speed counter-current chromatography. J Sep Sci 34:385–392
Cao X, Ito Y (2003) Supercritical fluid extraction of grape seed oil and subsequent separation of free fatty acids by high-speed countercurrent chromatography. J Chromatogr A 1021:117–124
Bousquet O, Le Goffi F (1995) Counter-current chromatographic separation of polyunsaturated fatty acids. J Chromatogr A 704:211–216
Kapp T, Vetter W (2009) Offline coupling of high-speed counter-current chromatography and gas chromatography/mass spectrometry generates a two-dimensional plot of toxaphene components. J Chromatogr A 1216:8391–9397
Vetter W, Kirres J, Bendig P (2011) Bromination of 2-methoxydiphenyl ether to an average of tetrabrominated 2-methoxydiphenyl ethers. Chemosphere 84:1117–1124
Du Q, Shu A, Ito Y (1996) Purification of fish oil ethyl esters by high-speed countercurrent chromatography using non-aqueous solvent systems. J Liq Chromatogr Relat Technol 19:1451–1457
Murayama W, Kosuge Y, Nakaya N, Nunogaki Y, Nunogaki K, Cazes J, Nunogaki H (1988) Preparative separation of unsaturated fatty acid esters by centrifugal partition chromatography (CPC). J Liq Chromatogr 11:283–300
Hauff S, Vetter W (2010) Exploring the fatty acids of vernix caseosa in form of their methyl esters by off-line coupling of non-aqueous reversed phase high performance liquid chromatography and gas chromatography coupled to mass spectrometry. J Chromatogr A 1217:8270–8278
Vetter W, Schröder M (2010) Concentrations of phytanic acid and pristanic acid are higher in organic than in conventional dairy products from the German market. Food Chem 119:746–752
Thurnhofer S, Vetter W (2005) A gas chromatography/electron ionization-mass spectrometry-selected ion monitoring method for determining the fatty acid pattern in food after formation of fatty acid methyl esters. J Agric Food Chem 53:8896–8903
Thurnhofer S, Vetter W (2006) Application of ethyl esters and d 3-methyl esters as internal standards for the gas chromatographic quantification of transesterified fatty acid methyl esters in food. J Agric Food Chem 54:3209–3214
Li D, Schröder M, Vetter W (2012) Isolation of 6,9,12,15-hexadecatetraenoic fatty acid (16:4n–1) methyl ester from transesterified fish oil by HSCCC. Chromatographia 75:1–6
Crexi VT, Mote ML, Monte ML, Pinto LAA (2012) Polyunsaturated fatty acid concentrates of carp oil: chemical hydrolysis and urea complexation. J Am Oil Chem Soc 89:32–334
Gámez-Meza N, Noriega-Rodríguez JA, Medina-Juárez LA, Ortega-García J, Monroy-Rivera J, Toro-Vázquez FJ, García HS, Angulo-Guerrero O (2003) Concentration of eicosapentaenoic acid and docosahexaenoic acid from fish oil by urea complexation. Food Res Int 36:721–727
Liu S, Zhang C, Hong P, Ji H (2006) Concentration of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) of tuna oil by urea complexation: optimization of progress parameters. J Food Eng 73:203–209
Chakraborty K, Vijayagopal P, Chakraborty RD, Vijayan KK (2010) Preparation of eicosapentaenoic acid concentrates from sardine oil by Bacillus circulans lipase. Food Chem 120:433–442
Ackman RG, Hooper SN (1968) Examination of isoprenoid fatty acids as distinguishing characteristics of specific marine oils with particular reference to whale oil. Comp Biochem Physiol 24:549–565
Hayes DG, Bengtsson YC, van Alstine JM, Setterwall F (1998) Urea complexation for the rapid, ecologically responsible fractionation of fatty acids from seed oil. J Am Oil Chem Soc 75:1403–1409
June Brown P, Mei G, Gibberd FB, Burston D, Mayne PD, McClinchy JE, Sidey M (1993) Diet and Refsum’s disease. The determination of phytanic acid and phytol in certain foods and the application of this knowledge to the choice of suitable convenience foods for patients with Refsum’s disease. J Human Nutr Diet 6:295–305
Josten H, Fieg G, Gutsche B, Johannisbauer W (2004) Harnstoffällung als konkurrenzfähiges Trennverfahren für schwierige Stofftrennungen in der Oleochemie. Chem Ing Tech 76:1700–1703
Schröder M, Vetter W (2011) GC/EI-MS determination of the diastereomer distribution of phytanic acid in food samples. J Am Oil Chem Soc 88:341–349
Jensen RG (1973) Composition of bovine milk lipids. J Am Oil Chem Soc 50:186–192
Thurnhofer S, Hottinger G, Vetter W (2007) Enantioselective determination of food-relevant anteiso fatty acids. Anal Chem 79:4696–4701
Hauff S, Vetter W (2010) Creation and evaluation of a two-dimensional contour plot of fatty acid methyl esters after off-line coupling of reversed-phase HPLC and GC/EI-MS. Anal Bioanal Chem 396:2695–2707
Haahti E, Nikkari T, Salmi A-M, Laaksonen A-L (1961) Fatty acids of vernix caseosa. Scand J Clin Lab Invest 13:70–73
Schogt JCM, Haverkamp Begemann P (1965) Isolation of 11-cyclohexylundecanoic acid from butter. J Lipid Res 6:466–470
Ohya H, Komai Y, Yamaguchi M (1986) Zinc tolerance of an isolated bacterium containing ω-cyclohexyl fatty acid. FEMS Microbiol Lett 34:257–260
Schlosser S, Vetter W (2011) Fatty acids and polar lipid content of cheese and mould-contaminated cheese. Eur J Lipid Sci Technol 113:469–478
Hauff S, Rilfors L, Hottinger G, Vetter W (2010) Structure and absolute configuration of an unsaturated anteiso fatty acid from Bacillus megaterium. J Chromatogr A 1217:1683–1687
The Lipid Library. http://lipidlibrary.aocs.org/
Chance DL, Gerhardt KO, Mawhinney TP (1998) Gas-liquid chromatography-mass spectrometry of hydroxyl fatty acids as their methyl esters tert-butyldimethylsilyl ethers. J Chromatogr A 793:91–98
Jenske R, Vetter W (2008) Gas chromatography electron-capture negative-ion mass spectrometry for the quantitative determination of 2- and 3-hydroxy fatty acids in bovine milk fat. J Agric Food Chem 56:5500–5505
Noble AC, Nawar WW (1971) Mass spectrometry of aldehyde esters. J Agric Food Chem 19:1039–1040
Yayli N, Kiran Z, Seymen H, Genc H (2001) Characterization of lipids and fatty acid methyl ester contents in leaves and roots of Crocus vallicola. Turk J Chem 25:391–395
Brechany EY, Christie WW (1992) Identification of the saturated oxo fatty acids in cheese. J Dairy Res 59:57–64
Brechany EY, Christie WW (1994) Identification of the unsaturated oxo fatty acids in cheese. J Dairy Res 62:111–115
Márquez-Ruiz G, Rodríguez-Pino V, de la Fuente MA (2011) Determination of 10-hydroxystearic, 10-ketostearic, 8-hydroxypalmitic, and 8-ketopalmitic acids in milk fat by solid-phase extraction plus gas chromatography-mass spectrometry. J Dairy Sci 94:4810–4819
Welke JE, Manfroi V, Zanus M, Lazarotto M, Alcaraz Zini C (2012) Characterization of the volatile profile of Brazilian Merlot wines through comprehensive two dimensional gas chromatography time-of-flight mass spectrometric detection. J Chromatogr A 1226:124–139
Bourgeois G, vivas N, Glories Y, Vitry C (1995) Identification paer spectrométrie de masse couplée à la chromatographie en phase gazeuse des produits de dégradation des hydorperoxydes de lácide linoléique. Sci Aliments 15:625–630
Glass RL, Krick TP, Sand DM, Rahn CH, Schlenk H (1975) Furanoid Fatty acids from fish lipids. Lipids 10:695–702
Guth H, Grosch W (1992) Furan fatty acids in butter and butter oil. Z Lebensm Unters Forsch 194:360–362
Vetter W, Laure S, Wendlinger C, Mattes A, Smith AWT, Knight DW (2012) Determination of furan fatty acids in food samples. J Am Oil Chem Soc 89:1501–1508. doi:10.1007/s11746-012-2038-6
Iverson JL, Eisner J, Firestone D (1965) Detection of trace fatty acids in fats and oils by urea fractionation and gas-liquid chromatography. J Am Oil Chem Soc 42:1063–1068
Precht D, Molkentin J (2003) Overestimation of linoleic acid and trans-C18:2 isomers in milk fats with emphasis on transΔ9, transΔ12-octadecadienoic acid. Milchwiss 58:30–34
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Schröder, M., Vetter, W. Detection of 430 Fatty Acid Methyl Esters from a Transesterified Butter Sample. J Am Oil Chem Soc 90, 771–790 (2013). https://doi.org/10.1007/s11746-013-2218-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-013-2218-z