Abstract
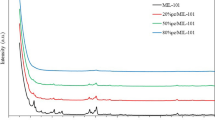
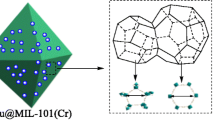
A CuAlCl4 doped metal organic framework, CuAlCl4@MIL-101, was prepared by introducing CuAlCl4 into the pores of MIL-101 for the selective adsorption of CO over N2. The CuAlCl4 molecules were evenly distributed into various pores sizes and did not change the intrinsic structure of the MIL-101. Isotherms for CO and N2 adsorption at 298 K showed that the CO capacity on CuAlCl4@MIL-101 was much higher than that on virgin MIL-101, whereas the N2 capacity decreased. The selectivity for CO over N2 improved from 4.64 to 31.5 at 298 K and 1 bar. The CuAlCl4@MIL-101 adsorbent displayed outstanding CO adsorption stability and the adsorbent could be regenerated by applying a simple vacuum of 4 mmHg.
Similar content being viewed by others
References
Singh B, Saxena A, Srivastava A K, Vijayaraghavan R. Impregnated carbon based catalyst for protection against carbon monoxide gas. Applied Catalysis B: Environmental, 2009, 88(3–4): 257–262
Saha D, Deng S. Adsorption equilibria and kinetics of carbon monoxide on zeolite 5Å, 13X, MOF-5, and MOF-177. Journal of Chemical & Engineering Data, 2009, 54(8): 2245–2250
Xie Y C, Zhang J P, Qiu J G, Tong X Z, Fu J P, Yang G, Yan H J, Tang Y Q. Zeolites modified by CuCl for separating CO from gas mixtures containing CO2. Adsorption-Journal of the International Adsorption Society, 1996, 3(1): 27–32
Noyes W. Military problems with aerosols and nonpersistent gases. National Defence Research Committee report division, 1946, 10
Beebe R A, Wildner E L. The heat of adsorption of carbon monoxide on copper I. Journal of the American Chemical Society, 1934, 56(3): 642–645
Kohl A, Riesenfeld F. Gas Purification. Texas: Gulf Publishing Company, 1985
Hogendoorn J A, Vanswaaij W P M, Versteeg G F. The absorption of carbon-monoxide in cosrb solutions-absorption rate and capacity. Chemical Engineering Journal and the Biochemical Engineering Journal, 1995, 59(3): 243–252
Keyworth D A, Haase D J, Walker D G, Turnbo R G. Low-cost carbon-monoxide using COSORB process. Abstracts of Papers of the American Chemical Society, 1978, 175(Mar): 66–66
Kasuya F, Tsuji T. High purity CO gas separation by pressure swing adsorption. Gas Separation & Purification, 1991, 5(4): 242–246
Yokoe J, Taki K, Aokata T T T, Kida M, Kasuya F. High-purity CO gas separation system by pressure swing adsorption method. In: Gas Separation Technology: Proceedings of the International Symposium on Gas Separation Technology
Hirai H, Komiyama M, Wada K. Active carbon-supported aluminum copper chloride as water-resistant carbon-monoxide adsorbent. Chemistry Letters, 1982, 7: 1025–1028
Ferey G, Mellot-Draznieks C, Serre C, Millange F, Dutour J, Surble S, Margiolaki I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science, 2005, 309(5743): 2040–2042
Wang X, Li H, Hou X J. Amine-functionalized metal organic framework as a highly selective adsorbent for CO2 over CO. Journal of Physical Chemistry C, 2012, 116(37): 19814–19821
Yang J F, Zhao Q, Li J P, Dong J X. Synthesis of metal-organic framework MIL-101 in TMAOH-Cr(NO3)(3)-H2BDC-H2O and its hydrogen-storage behavior. Microporous and Mesoporous Materials, 2010, 130(1–3): 174–179
Hong D Y, Hwang Y K, Serre C, Ferey G, Chang J S. Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis. Advanced Functional Materials, 2009, 19(10): 1537–1552
Hwang Y K, Hong D Y, Chang J S, Jung S H, Seo Y K, Kim J, Vimont A, Daturi M, Serre C, Ferey G. Amine grafting on coordinatively unsaturated metal centers of MOFs: Consequences for catalysis and metal encapsulation. Angewandte Chemie International Edition, 2008, 47(22): 4144–4148
Henschel A, Gedrich K, Kraehnert R, Kaskel S. Catalytic properties of MIL-101. Chemical Communications, 2008, 35: 4192–4194
Xiang Z H, Hu Z, Yang W T, Cao D P. Lithium doping on metalorganic frameworks for enhancing H-2 Storage. International Journal of Hydrogen Energy, 2012, 37(1): 946–950
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Li, C., Meng, F. et al. CuAlCl4 doped MIL-101 as a high capacity CO adsorbent with selectivity over N2 . Front. Chem. Sci. Eng. 8, 340–345 (2014). https://doi.org/10.1007/s11705-014-1438-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-014-1438-6