Abstract
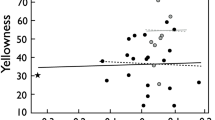
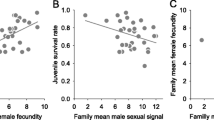
Genetic theories of sexual selection predict that most ornamental secondary sexual traits provide reliable indication of the genetic quality of their bearers. Accordingly, also the offspring of mates with elaborate mating display should perform better than those of less conspicuous counterparts. In this study, we used Arctic charr (Salvelinus alpinus) as a model species to investigate whether the variation in a carotenoid-based red breeding coloration (a sexually dichromatic trait) in different sexes would reflect differences in individual genetic variability, one measure of individual quality, and/or indirectly, be manifested in variation in the offspring’s early viability and growth. We created maternal half-sibling families by artificially fertilizing the eggs with milt from bright- and pale-coloured males and then held the resulting progenies under identical hatchery conditions. The expression of red coloration among parental fish was not associated with their genetic diversity estimates in either sex nor did offspring sired by bright males consistently differ in terms of embryo survival or endogenous growth efficiency from offspring sired by pale males. By contrast, maternal effects were notably strong and, additionally, the degree of female coloration was negatively linked to their reproductive potential. The more intensely coloured females had a smaller relative fecundity and they also produced offspring of lower viability, implying a significant trade-off in resource allocation between ornamentation and offspring. Our results indicate that the red breeding ornamentation of Arctic charr is likely to be informative rather among females than males when the reproductive quality is predicted on grounds of the number of offspring produced. Nevertheless, this study does not support the direct selection hypothesis in explaining the evolution of female ornamentation, but rather suggests that the less intense coloration of female charr compared to males may reflect inter-sexual differences in the trade-off between natural and sexual selection.



Similar content being viewed by others
References
Ahmadi, M. R., Bazyar, A. A., Safi, S., Ytrestoyl, T., & Bjerkeng, B. (2006). Effects of dietary astaxanthin supplementation on reproductive characteristics of rainbow trout (Oncorhynchus mykiss). Journal of Applied Ichthyology, 22(5), 388–394.
Amos, W., Worthington-Wilmer, J., Fullard, K., Burg, T. M., Croxall, J. P., Bloch, D., et al. (2001). The influence of paternal relatedness on reproductive success. Proceedings of the Royal Society of London Series B, 268(1480), 2021–2027.
Amundsen, T. (2000). Why are female birds ornamented? Trends in Ecology & Evolution, 15(7), 149–155.
Andersson, M. (1994). Sexual selection. Princeton: Princeton University Press.
Aparicio, J. M., Cordero, P. J., & Veiga, J. P. (2001). A test of the hypothesis of mate choice based on heterozygosity in the spotless starling. Animal Behaviour, 62(5), 1001–1006.
Aparicio, J. M., Ortego, J., & Cordero, P. J. (2006). What should we weigh to estimate heterozygosity, alleles or loci? Molecular Ecology, 15(14), 4659–4665.
Baeta, R., Faivre, B., Motreuil, S., Gaillard, M., & Moreau, J. (2008). Carotenoid trade-off between parasitic resistance and sexual display: An experimental study in the blackbird (Turdus merula). Proceedings of the Royal Society of London Series B, 275(1633), 427–434.
Bakker, T. C. M., & Mundwiler, B. (1994). Female mate choice and male red coloration in a natural 3-spined stickleback (Gasterosteus aculeatus) population. Behavioral Ecology, 5(1), 74–80.
Barber, I., Arnott, S. A., Braithwaite, V., Andrew, J., & Huntingford, F. A. (2001). Indirect fitness consequences of mate choice in sticklebacks: Offspring of brighter males grow slowly but resist parasitic infections. Proceedings of the Royal Society of London Series B, 268(1462), 71–76.
Barber, I., Arnott, S. A., Braithwaite, V., Andrew, J., Mullen, W., & Huntingford, F. A. (2000). Carotenoid-based sexual coloration and body condition in nestling male sticklebacks. Journal of Fish Biology, 57(3), 777–790.
Blount, J. D. (2004). Carotenoids and life-history evolution in animals. Archives of Biochemistry and Biophysics, 430(1), 10–15.
Blount, J. D., Houston, D. C., & Møller, A. P. (2000). Why egg yolk is yellow? Trends in Ecology & Evolution, 15(2), 47–49.
Blount, J. D., Metcalfe, N. B., Arnold, K. E., Surai, P. F., Devevey, G. L., & Monaghan, P. (2003). Neonatal nutrition, adult antioxidant defences and sexual attractiveness in the zebra finch. Proceedings of the Royal Society of London Series B, 270(1525), 1691–1696.
Brown, J. L. (1997). A theory of mate choice based on heterozygosity. Behavioral Ecology, 8(1), 60–65.
CIE (International Commission on Illumination). (1986). Colorimetry. Vienna: CIE Publication No. 15.2.
Cotton, S., Fowler, K., & Pomiankowski, A. (2004). Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proceedings of the Royal Society of London Series B, 271(1541), 771–783.
Craig, J. K., Foote, C. J., & Wood, C. C. (2005). Countergradient variation in carotenoid use between sympatric morphs of sockeye salmon (Oncorhynchus nerka) exposes nonanadromous hybrids in the wild by their mismatched spawning colour. Biological Journal of the Linnean Society, 84(2), 287–305.
Crozier, G. F. (1970). Tissue carotenoids in prespawning and spawning sockeye salmon (Oncorhynchus nerka). Journal of the Fisheries Research Board of Canada, 27, 973–975.
Eilertsen, E. M., Bårdsen, B.-J., Liljedal, S., Rudolfsen, G., & Folstad, I. (2009). Experimental evidence for paternal effects on offspring growth rate in Arctic charr (Salvelinus alpinus). Proceedings of the Royal Society of London Series B, 276(1654), 129–136.
Elvingson, P., & Nilsson, J. (1994). Phenotypic and genetic parameters of body and compositional traits in Arctic charr, Salvelinus alpinus (L.). Aquaculture Research, 25(7), 677–685.
Fitzpatrick, S., Berglund, A., & Rosenqvist, G. (1995). Ornaments or offspring: Costs to reproductive success restrict sexual selection processes. Biological Journal of the Linnean Society, 55(3), 251–260.
Foerster, K., Delhey, K., Johnsen, A., Lifjeld, J. T., & Kempenaers, B. (2003). Females increase offspring heterozygosity and fitness through extra-pair matings. Nature, 425(6959), 714–717.
Folstad, I., Hope, A. M., Karter, A., & Skorping, A. (1994). Sexually selected color in male sticklebacks: A signal of both parasite exposure and parasite resistance? Oikos, 69(3), 511–515.
Fromhage, L., Kokko, H., & Reid, J. M. (2009). Evolution of mate choice for genome-wide heterozygosity. Evolution, 63(3), 684–694.
George, S. B., Lawrence, J. M., Lawrence, A. L., Smiley, J., & Plank, L. (2001). Carotenoids in the adult diet enhance egg and juvenile production in the sea urchin Lytechinus variegatus. Aquaculture, 199(3–4), 353–369.
Gladbach, A., Gladbach, D. J., Kempenaers, B., & Quillfeldt, P. (2010). Female-specific colouration, carotenoids and reproductive investment in a dichromatic species, the upland goose Chloephaga picta leucoptera. Behavioral Ecology and Sociobiology, 64(11), 1779–1789.
Hadfield, J. D., & Owens, I. P. F. (2006). Strong environmental determination of a carotenoid-based plumage trait is not mediated by carotenoid availability. Journal of Evolutionary Biology, 19(4), 1104–1114.
Hamon, T. R., & Foote, C. J. (2005). Concurrent natural and sexual selection in wild male sockeye salmon, Oncorhynchus nerka. Evolution, 59(5), 1104–1118.
Hatlen, B., Arnesen, A. M., Jobling, M., Siikavuopio, S., & Bjerkeng, B. (1997). Carotenoid pigmentation in relation to feed intake, growth and social interactions in Arctic charr, Salvelinus alpinus (L.), from two anadromous strains. Aquaculture Nutrition, 3(3), 189–199.
Hatlen, B., Jobling, M., & Bjerkeng, B. (1998). Relationships between carotenoid concentration and colour of fillets of Arctic chair, Salvelinus alpinus (L.), fed astaxanthin. Aquaculture Research, 29(3), 191–202.
Hughes, K. A., Rodd, F. H., & Reznick, D. N. (2005). Genetic and environmental effects on secondary sex traits in guppies (Poecilia reticulata). Journal of Evolutionary Biology, 18(1), 35–45.
Hunt, J., Bussière, L. F., Jennions, M. D., & Brooks, R. (2004). What is genetic quality? Trends in Ecology & Evolution, 19(6), 329–333.
Janhunen, M., Piironen, J., & Peuhkuri, N. (2010). Parental effects on embryonic viability and growth in Arctic charr Salvelinus alpinus at two incubation temperatures. Journal of Fish Biology, 76(10), 2558–2570.
Janhunen, M., Rudolfsen, G., Kekäläinen, J., Figenschou, L., Peuhkuri, N., & Kortet, R. (2009). Spawning coloration and sperm quality in a large lake population of Arctic charr (Salmonidae: Salvelinus alpinus L.). Biological Journal of the Linnean Society, 98(4), 794–802.
Kamler, E. (2008). Resource allocation in yolk-feeding fish. Reviews in Fish Biology and Fisheries, 18(2), 143–200.
Kekäläinen, J., Vallunen, J. A., Primmer, C. R., Rättyä, J., & Taskinen, J. (2009). Signals of major histocompatibility complex overdominance in a wild salmonid population. Proceedings of the Royal Society of London Series B, 276(1670), 3133–3140.
Kempenaers, B. (2007). Mate choice and genetic quality: A review of the heterozygosity theory. Advances in the Study of Behaviour, 37, 189–278.
Kokko, H., Brooks, R., Jennions, M. D., & Morley, J. (2003). The evolution of mate choice and mating biases. Proceedings of the Royal Society of London Series B, 270(1515), 653–664.
Kortet, R., Vainikka, A., Rantala, M. J., Myntti, J., & Taskinen, J. (2004). In vitro embryo survival and early viability of larvae in relation to male sexual ornaments and parasite resistance in roach, Rutilus rutilus L. Journal of Evolutionary Biology, 17(6), 1337–1344.
Kraaijeveld, K., Kraaijeveld-Smit, F. J. L., & Komdeur, J. (2007). The evolution of mutual ornamentation. Animal Behaviour, 74(4), 657–677.
Lande, R. (1980). Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution, 34(2), 292–305.
LeBas, N. R. (2006). Female finery is not for males. Trends in Ecology & Evolution, 21(4), 170–173.
Lehmann, L., Keller, L. F., & Kokko, H. (2006). Mate choice evolution, dominance effects, and the maintenance of genetic variation. Journal of Theoretical Biology, 244(2), 282–295.
MacDougall, A. K., & Montgomerie, R. (2003). Assortative mating by carotenoid-based plumage colour: A quality indicator in American goldfinches, Garduelis tristis. Die Naturwissenschaften, 90(10), 464–467.
Marshall, R. C., Buchanan, K. L., & Catchpole, C. K. (2003). Sexual selection and individual genetic diversity in a songbird. Proceedings of the Royal Society of London Series B, 270(Suppl 2), S248–S250.
Måsvær, M., Liljedal, S., & Folstad, I. (2004). Are secondary sexual traits, parasites and immunity related to variation in primary sex traits in the Arctic charr? Proceedings of the Royal Society of London Series B, 271(Suppl), S40–S42.
Mays, H. L., & Hill, G. E. (2004). Choosing mates: Good genes versus genes that are a good fit. Trends in Ecology & Evolution, 19(10), 554–559.
McGraw, K. J. (2005). The antioxidant function of many animal pigments: Are there consistent health benefits of sexually selected colourants? Animal Behaviour, 69(4), 757–764.
McGraw, K. J., Adkins-Regan, E., & Parker, R. S. (2005a). Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colourful songbird. Die Naturwissenschaften, 92(8), 375–380.
McGraw, K. J., & Ardia, D. R. (2003). Carotenoids, immunocompetence, and the information content of sexual colors: An experimental test. The American Naturalist, 162(6), 704–712.
McGraw, K. J., Hill, G. E., & Parker, R. S. (2005b). The physiological costs of being colourful: Nutritional control of carotenoid utilization in the American goldfinch, Carduelis tristis. Animal Behaviour, 69(3), 653–660.
Milinski, M., & Bakker, T. C. M. (1990). Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature, 344(6264), 330–333.
Mitton, J. B. (1993). Enzyme heterozygosity, metabolism and developmental stability. Genetica, 89(1–3), 47–65.
Møller, A. P., & Alatalo, R. (1999). Good-genes effects in sexual selection. Proceedings of the Royal Society of London Series B, 266(1414), 85–91.
Møller, A. P., Biard, C., Blount, J. D., Houston, D. C., Ninni, P., Saino, N., et al. (2000). Carotenoid-dependent signals: Indicators of foraging efficiency, immunocompetence or detoxification ability? Avian and Poultry Biological Reviews, 11, 137–159.
Mougeot, F., Pérez-Rodríguez, L., Martínez-Padilla, J., Leckie, F., & Redpath, S. M. (2007). Parasites, testosterone and honest carotenoid-based signalling of health. Functional Ecology, 21(5), 886–898.
Müller, G., & Ward, P. I. (1995). Parasitism and heterozygosity influence the secondary sexual characters of the European minnow, Phoxinus phoxinus (L.) (Cyprinidae). Ethology, 100(4), 309–319.
Nagler, J. J., Parsons, J. E., & Cloud, J. G. (2000). Single pair mating indicates maternal effects on embryo survival in rainbow trout, Oncorhynchus mykiss. Aquaculture, 184(1), 177–183.
Neff, B. D., & Pitcher, T. E. (2005). Genetic quality and sexual selection: An integrated framework for good genes and compatible genes. Molecular Ecology, 14(1), 19–38.
Nordeide, J. T., Mohus, Å., Nicolaisen, O., Volden, R., & Egeland, E. S. (2008). Offspring or ornaments? Is carotenoid-based ornamentation in female Arctic charr, Salvelinus alpinus (L.), condition-dependent and traded off against offspring? Ecology of Freshwater Fish, 17(2), 328–339.
Nordeide, J. T., Rudolfsen, G., & Egeland, E. S. (2006). Ornaments or offspring? Female sticklebacks (Gasterosteus aculeatus L.) trade off carotenoids between spines and eggs. Journal of Evolutionary Biology, 19(2), 431–439.
Olson, V. A., & Owens, I. P. F. (1998). Costly sexual signals: Are carotenoids rare, risky or required? Trends in Ecology & Evolution, 13(12), 510–514.
Perry, G. M. L., Audet, C., Laplatte, B., & Bernantchez, L. (2004). Shifting patterns in genetic control at the embryo-alevin boundary in brook charr. Evolution, 58(9), 2002–2012.
Pike, T. W., Blount, J. D., Lindström, J., & Metcalfe, N. B. (2007). Availability of non-carotenoid antioxidants affects the expression of a carotenoid-based sexual ornament. Biology Letters, 3(4), 353–356.
Pitcher, T. E., & Neff, B. D. (2007). Genetic quality and offspring performance in Chinook salmon: Implications for supportive breeding. Conservation Genetics, 8(3), 607–616.
Primmer, C. R., Landry, P.-A., Ranta, E., Merilä, J., Piironen, J., Tiira, K., et al. (2003). Prediction of offspring fitness based on parental genetic diversity in endangered salmonid populations. Journal of Fish Biology, 63(4), 909–927.
Puurtinen, M., Ketola, T., & Kotiaho, J. S. (2009). The good-genes and compatible-genes benefits of mate choice. The American Naturalist, 174(5), 741–751.
Reid, J. M., Arcese, P., Cassidy, A. L. E. V., Marr, A. B., Smith, J. N. M., & Keller, L. F. (2005). Hamilton and Zuk meet heterozygosity? Song repertoire size signals inbreeding and immunity in song sparrows (Melospiza melodia). Proceedings of the Royal Society of London Series B, 272(1562), 481–487.
Rosen, R. F., & Tarvin, K. A. (2006). Sexual signals of the male American Goldfinch. Ethology, 112(10), 1008–1019.
Rudolfsen, G., Figenschou, L., Folstad, I., Nordeide, J. T., & Søreng, E. (2005). Potential fitness benefits from mate selection in the Atlantic cod (Gadus morhua). Journal of Evolutionary Biology, 18(1), 172–179.
Salze, G., Tocher, D. R., Roy, W. J., & Robertson, D. A. (2005). Egg quality determinants in cod (Gadus morhua L.): Egg performance and lipids in eggs from farmed and wild broodstock. Aquaculture Research, 36(15), 1488–1499.
Sawanboonchun, J., Roy, W. J., Robertson, D. A., & Bell, J. G. (2008). The impact of dietary supplementation with astaxanthin on egg quality in Atlantic cod broodstock (Gadus morhua L.). Aquaculture, 283, 97–101.
Scalia, S., Isaksen, M., & Francis, G. W. (1989). Carotenoids of the Arctic charr, Salvelinus alpinus (L.). Journal of Fish Biology, 34(6), 969–970.
Sheldon, B. C. (2000). Differential allocation: Tests, mechanisms and implications. Trends in Ecology & Evolution, 15(10), 397–402.
Sigurjónsdóttir, H., & Gunnarson, K. (1989). Alternative mating tactics of Arctic charr. Salvelinus alpinus, in Thingvallavatn, Iceland. Environmental Biology of Fishes, 26(3), 159–176.
Skarstein, F., & Folstad, I. (1996). Sexual dichromatism and the immunocompetence handicap: An observational approach using Arctic charr. Oikos, 76(2), 359–367.
Skarstein, F., Folstad, I., & Rønning, H. P. (2005). Spawning colouration, parasites and habitat selection in Salvelinus alpinus: Initiating speciation by sexual selection? Journal of Fish Biology, 67(4), 969–980.
Slate, J., David, P., Dodds, K. G., Veenvliet, B. A., Glass, B. C., Broad, T. E., et al. (2004). Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: Theoretical implications and empirical data. Heredity, 93(3), 255–265.
Stuart-Fox, D. M., & Ord, T. J. (2004). Sexual selection, natural selection and the evolution of dimorphic coloration and ornamentation in agamid lizards. Proceedings of the Royal Society of London Series B, 271(1554), 2249–2255.
Tomkins, J. L., Radwan, J., Kotiaho, J. S., & Tregenza, T. (2004). Genic capture and resolving the lek paradox. Trends in Ecology & Evolution, 19(6), 323–328.
Torrissen, O. J. (1984). Pigmentation of salmonids—Effects of carotenoids in eggs and startfeeding diet on survival and growth rate. Aquaculture, 43(1–3), 185–193.
Tyndale, S. T., Letcher, R. J., Heath, J. W., & Heath, D. D. (2008). Why are salmon eggs red? Egg carotenoids and early life survival of Chinook salmon (Oncorhynchus tshawytscha). Evolutionary Ecology Research, 10(8), 1187–1199.
van Oosterhout, C., Trigg, R. E., Carvalho, G. R., Magurran, A. E., Hauser, L., & Shaw, P. W. (2003). Inbreeding depression and genetic load of sexually selected traits: How guppy lost its spots. Journal of Evolutionary Biology, 16(2), 273–281.
Watson, N. L., & Simmons, L. W. (2010). Reproductive competition promotes the evolution of female weaponry. Proceedings of the Royal Society of London Series B, 277(1690), 2035–2040.
Wedekind, C., Evanno, G., Urbach, D., Jacob, A., & Müller, R. (2008a). ‘Good genes’ and ‘compatible genes’ effects in an Alpine whitefish and the information content of breeding tubercles over the course of the spawning season. Genetica, 134(1), 21–30.
Wedekind, C., Jacob, A., Evanno, G., Nusslé, S., & Müller, R. (2008b). Viability of brown trout embryos positively linked to melanin-based but negatively to carotenoid-based colours of their fathers. Proceedings of the Royal Society of London Series B, 275(1644), 1737–1744.
Wedekind, C., Müller, R., & Spicher, H. (2001). Potential genetic benefits of mate selection in whitefish. Journal of Evolutionary Biology, 14(6), 980–986.
Wedell, N., Kvarnemo, C., Lessells, C. M., & Tregenza, T. (2006). Sexual conflict and life histories. Animal Behaviour, 71(5), 999–1011.
Zahavi, A. (1975). Mate selection: A selection for a handicap. Journal of Theoretical Biology, 53(1), 205–214.
Acknowledgments
This study was funded by the Ministry of Agriculture and Forestry (310613), Jenny and Antti Wihuri Foundation, and by Finnish Game and Fisheries Research Institute. We wish to thank Saimaa Fisheries Research and Aquaculture (FGFRI) for providing the experimental fish and facilities and especially Tapani Heikkinen, Maija Hyttinen and Jorma Sorjonen for their assistance in the study. Two anonymous reviewers are thanked for their constructive suggestions on a previous draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janhunen, M., Peuhkuri, N., Primmer, C.R. et al. Does Breeding Ornamentation Signal Genetic Quality in Arctic charr, Salvelinus alpinus?. Evol Biol 38, 68–78 (2011). https://doi.org/10.1007/s11692-010-9100-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-010-9100-9