Abstract
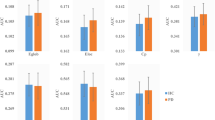
Although previous studies reported altered topology of brain functional networks in patients with Parkinson’s disease (PD), the modular organization of brain functional networks in PD patients remains largely unknown. Using the resting-state functional MRI (R-fMRI) and graph theory, we examined the modular organization of brain functional networks in 32 unmedicated patients with early-to-mid motor stage PD and 31 healthy controls. Compared to the controls, the PD patients tended to show decreased integrity and segregation, both within and between modules. This was inferred by significantly increased intra-modular characteristic path length (L p) within four modules: mPFC, SN, SMN, and FPN, decreased inter-modular functional connectivity (FC) between mPFC and SN, SMN, and VN, and decreased intra-modular clustering in the PD patients. Intra-modular characteristic path length within the mPFC showed significantly positive correlation with general cognitive ability in the PD group. Receiver operating characteristic (ROC) analysis revealed that FC between mPFC and SN had the highest significant accuracy in differentiating the patients from the controls. Our findings may provide new insight in understanding the pathological changes that underlie impairment in cognition and movement in Parkinson’s disease.





Similar content being viewed by others
Abbreviations
- mPFC:
-
Medial prefrontal cortex
- SN:
-
Salience network
- FPN:
-
Fronto-parietal network
- SMN:
-
Somatomotor network
- VN:
-
Visual network
- pCER:
-
Posterior cerebellum
- DMN:
-
Default mode network
References
Achard, S., Salvador, R., Whitcher, B., Suckling, J., & Bullmore, E. (2006). A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. The Journal of Neuroscience, 26(1), 63–72.
Alexander-Bloch, A. F., Gogtay, N., Meunier, D., Birn, R., Clasen, L., Lalonde, F., Lenroot, R., Giedd, J., & Bullmore, E. T. (2010). Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Frontiers in Systems Neuroscience, 4(147), 1–16.
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113.
Ashburner, J., & Friston, K. J. (2000). Voxel-based morphometry—the methods. NeuroImage, 11(6), 805–821.
Baggio, H. C., Sala-Llonch, R., Segura, B., Marti, M. J., Valldeoriola, F., Compta, Y., Tolosa, E., & Junqué, C. (2014). Functional brain networks and cognitive deficits in Parkinson’s disease. Human Brain Mapping, 35(9), 4620–4634.
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B: Statistical Methodology, 57, 289–300.
Biundo, R., Calabrese, M., Weis, L., Facchini, S., Ricchieri, G., Gallo, P., & Antonini, A. (2013). Anatomical correlates of cognitive functions in early Parkinson’s disease patients. PloS One, 88, e64222.
Boller, F., Passafiume, D., Keefe, N. C., Rogers, K., Morrow, L., & Kim, Y. (1984). Visuospatial impairment in Parkinson’s disease: role of perceptual and motor factors. Archives of Neurology, 41(5), 485–490.
Borghammer, P., Cumming, P., Østergaard, K., Gjedde, A., Rodell, A., Bailey, C. J., & Vafaee, M. S. (2012). Cerebral oxygen metabolism in patients with early Parkinson’s disease. Journal of the Neurological Sciences, 313(1), 123–128.
Braak, H., Tredici, D. K., Rüb, U., de Vos, A. I. R., Steur, N. H. J. E., & Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging, 24(2), 197–211.
Bullmore, E. T., & Bassett, D. S. (2011). Brain graphs: graphical models of the human brain connectome. Annual Review of Clinical Psychology, 7(7), 113–140.
Bullmore, E., & Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews. Neuroscience, 10(3), 186–198.
Cardoso, E. F., Maia, F. M., Fregni, F., Myczkowski, M. L., Melo, L. M., Sato, J. R., Marcolin, M. A., Rigonatti, S. P., Cruz Jr., A. C., & Barbosa, E. R. (2009). Depression in Parkinson’s disease: convergence from voxel-based morphometry and functional magnetic resonance imaging in the limbic thalamus. NeuroImage, 47(2), 467–472.
Chen, Z. J., He, Y., Rosa-Neto, P., Gong, G., & Evans, A. C. (2011b). Age-related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. NeuroImage, 56(1), 235–245.
Chen, G., Ward, B. D., Xie, C., Li, W., Wu, Z., Jones, J. L., Franczak, M., Antuono, P., & Li, S.-J. (2011a). Classification of Alzheimer disease, mild cognitive impairment, and normal cognitive status with large-scale network analysis based on resting-state functional MR imaging. Radiology, 259(1), 213–221.
Chen, Z. J., He, Y., Rosa-Neto, P., Germann, J., & Evans, A. C. (2008). Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cerebral Cortex, 18(10), 2374–2381.
Chen, G., Zhang, H.-Y., Xie, C., Chen, G., Zhang, Z.-J., Teng, G.-J., & Li, S.-J. (2013). Modular reorganization of brain resting state networks and its independent validation in Alzheimer’s disease patients. Frontiers in Human Neuroscience, 7, 456.
Christopher, L., Marras, C., Duff-Canning, S., Koshimori, Y., Chen, R., Boileau, I., Segura, B., Monchi, O., Lang, A. E., & Rusjan, P. (2014). Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson’s disease with mild cognitive impairment. Brain, 137(Pt 2), 565–575.
Cruse, D., Chennu, S., Chatelle, C., Bekinschtein, T. A., Fernandez-Espejo, D., Pickard, J. D., Laureys, S., & Owen, A. M. (2011). Bedside detection of awareness in the vegetative state: a cohort study. Lancet, 378(9809), 2088–2094.
Dai, Z., Yan, C., Li, K., Wang, Z., Wang, J., Cao, M., Lin, Q., Shu, N., Xia, M., & Bi, Y. (2014). Identifying and Mapping Connectivity Patterns of Brain Network Hubs in Alzheimer’s Disease. Cerebral Cortex, 2014 epub bhu246.
Damoiseaux, J., Beckmann, C., Arigita, E. S., Barkhof, F., Scheltens, P., Stam, C., Smith, S., & Rombouts, S. (2008). Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex, 18(8), 1856–1864.
Doyon, J., Gaudreau, D., Castonguay, M., Bedard, P., Bédard, F., & Bouchard, J. (1997). Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain and Cognition, 34(2), 218–245.
Dubbelink, K. T. O., Hillebrand, A., Stoffers, D., Deijen, J. B., Twisk, J. W., Stam, C. J., & Berendse, H. W. (2013). Disrupted brain network topology in Parkinson’s disease: a longitudinal magnetoencephalography study. Brain, 137(1), 197–207.
Euston, D. R., Gruber, A. J., McNaughton, B. L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron, 76(6), 1057–1070.
Fearnley, J. M., & Lees, A. J. (1991). Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain, 114(5), 2283–2301.
Ferri, F., Frassinetti, F., Ardizzi, M., Costantini, M., & Gallese, V. (2012). A sensorimotor network for the bodily self. Journal of Cognitive Neuroscience, 24(7), 1584–1595.
Foti, N. J., Hughes, J. M., & Rockmore, D. N. (2011). Nonparametric sparsification of complex multiscale networks. PloS One, 6(2), e16431.
Fox, M. D., Zhang, D., Snyder, A. Z., & Raichle, M. E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101(6), 3270–3283.
Friston, K. J., Williams, S., Howard, R., Frackowial, R. S., & Turner, R. (1996). Movement related effects in fMRI time series. Magnetic Resonance in Medicine, 35(3), 346–355.
Gorges, M., Müller, H. P., Lulé, D., Consortium, L., Pinkhardt, E. H., Ludolph, A. C., & Kassubek, J. (2015). To rise and to fall: functional connectivity in cognitively normal and cognitively impaired patients with Parkinson’s disease. Neurobiology of Aging, 36(4), 1727–1735.
Göttlich, M., Münte, T. F., Heldmann, M., Kasten, M., Hagenah, J., & Krämer, U. M. (2013). Altered resting state brain networks in Parkinson’s disease. PloS One, 8(10), e77336.
Guimera, R., & Amaral, L. A. N. (2005). Functional cartography of complex metabolic networks. Nature, 433(7028), 895–900.
de Haan, W., van der Flier, W. M., Koene, T., Smits, L., Scheltens, P., & Stam, C. J. (2012). Disrupted modular brain dynamics reflect cognitive dysfunction in Alzheimer’s disease. NeuroImage, 59(4), 3085–3093.
Hawkes, C. H., Tredici, K. D., & Braak, H. (2009). Parkinson’s disease. Annals of the New York Academy of Sciences, 1170(1), 615–622.
He, Y., Wang, J., Wang, L., Chen, Z. J., Yan, C., Yang, H., Tang, H., Zhu, C., Gong, Q., & Zang, Y. (2009). Uncovering intrinsic modular organization of spontaneous brain activity in humans. PloS One, 4, e5226.
Helmich, R. C., Derikx, L. C., Bakker, M., Scheeringa, R., Bloem, B. R., & Toni, I. (2010). Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cerebral Cortex, 20(5), 1175–1186.
Hoehn, M. M., & Yahr, M. D. (1998). Parkinsonism: onset, progression, and mortality. Neurology, 50(2), 318–318.
Jenkins, A. C., & Mitchell, J. P. (2011). Medial prefrontal cortex subserves diverse forms of self-reflection. Social Neuroscience, 6(3), 211–218.
Jucker, M., & Walker, L. C. (2013). Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature, 501(7465), 45–51.
Kikuchi, A., Takeda, A., Kimpara, T., Nakagawa, M., Kawashima, R., Sugiura, M., Kinomura, S., Fukuda, H., Chida, K., & Okita, N. (2001). Hypoperfusion in the supplementary motor area, dorsolateral prefrontal cortex and insular cortex in Parkinson’s disease. Journal of the Neurological Sciences, 193(1), 29–36.
Kurani, A. S., Seidler, R. D., Burciu, R. G., Comella, C. L., Corcos, D. M., Okun, M. S., MacKinnon, C. D., & Vaillancourt, D. E. (2015). Subthalamic nucleus—sensorimotor cortex functional connectivity in de novo and moderate Parkinson’s disease. Neurobiology of Aging, 36(1), 462–469.
Lebedev, A. V., Westman, E., Simmons, A., Lebedeva, A., Siepel, F. J., Pereira, J. B., & Aarsland, D. (2014). Large-scale resting state network correlates of cognitive impairment in Parkinson’s disease and related dopaminergic deficits. Frontiers in Systems Neuroscience, 8, 45.
Long, D., Wang, J., Xuan, M., Gu, Q., Xu, X., Kong, D., & Zhang, M. (2012). Automatic classification of early Parkinson’s disease with multi-modal MR imaging. PloS One, 7, e47714.
Luo, C. Y., Guo, X. Y., Song, W., Chen, Q., Cao, B., Yang, J., Gong, Q. Y., & Shang, H.-F. (2015). Functional connectome assessed using graph theory in drug-naive Parkinson’s disease. Journal of Neurology, 262(60), 1557–1567.
Mesulam, M.-M. (1998). From sensation to cognition. Brain, 121(6), 1013–1052.
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B., & Bandettini, P. A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage, 44(3), 893–905.
Newman, M. E. (2004). Analysis of weighted networks. Physical Review. E, Statistical, Nonlinear, and Soft Matter Physics, 70(5), 056131.
Newman, M. E. J. (2006). Finding community structure in networks using the eigenvectors of matrices. Physical Review E, 74(3), 036104.
Polito, C., Berti, V., Ramat, S., Vanzi, E., De Cristofaro, M. T., Pellicanò, G., Mungai, F., Marini, P., Formiconi, A. R., & Sorbi, S. (2012). Interaction of caudate dopamine depletion and brain metabolic changes with cognitive dysfunction in early Parkinson’s disease. Neurobiology of Aging, 33(1), 206. e229–206. e239.
Pont-Sunyer, C., Hotter, A., Gaig, C., Seppi, K., Compta, Y., Katzenschlager, R., Mas, N., Hofeneder, D., Brücke, T., & Bayés, A. (2014). The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD Study). Movement Disorders, 30(2), 229–237.
Power, J. D., Schlaggar, B. L., & Petersen, S. E. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–551.
Putcha, D., Ross, R. S., Cronin-Golomb, A., Janes, A. C., & Stern, C. E. (2015). Altered intrinsic functional coupling between core neurocognitive networks in Parkinson’s disease. Neuroimage Clin, 7, 449–455.
Pyatigorskaya, N., Gallea, C., Garcia-Lorenzo, D., Vidailhet, M., & Lehéricy, S. (2013). A review of the use of magnetic resonance imaging in Parkinson’s disease. Ther. Adv Neurol Disord, 2014, 7(4), 206–220.
Radebaugh, T., & Khachaturian, Z. (1998). Consensus report of the Working Group on: molecular and biochemical markers of Alzheimer’s disease. Neurobiology of Aging, 19(2), 109–116.
Rae, C. L., Correia, M. M., Altena, E., Hughes, L. E., Barker, R. A., & Rowe, J. B. (2012). White matter pathology in Parkinson’s disease: the effect of imaging protocol differences and relevance to executive function. NeuroImage, 62(3), 1675–1684.
Raichle, M. E., & Snyder, A. Z. (2007). A default mode of brain function: a brief history of an evolving idea. NeuroImage, 37(4), 1083–1090.
Rektorova, I., Biundo, R., Marecek, R., Weis, L., Aarsland, D., & Antonini, A. (2014). Grey matter changes in cognitively impaired Parkinson’s disease patients. PloS One, 9(1), e85595.
Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. NeuroImage, 52(3), 1059–1069.
Satterthwaite, T. D., Wolf, D. H., Loughead, J., Ruparel, K., Elliott, M. A., Hakonarson, H., Gur, R. C., & Gur, R. E. (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage, 60(1), 623–632.
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., Reiss, A. L., & Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27(9), 2349–2356.
Skidmore, F., Korenkevych, D., Liu, Y., He, G., Bullmore, E., & Pardalos, P. M. (2011). Connectivity brain networks based on wavelet correlation analysis in Parkinson fMRI data. Neuroscience Letters, 499(1), 47–51.
Somerville, L. H., Jones, R. M., Ruberry, E. J., Dyke, J. P., Glover, G., & Casey, B. (2013). The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science, 24(8), 1554–1562.
Sporns, O. (2011). The non-random brain: efficiency, economy, and complex dynamics. Frontiers in Computational Neuroscience, 5(5), 1–13.
Sporns, O., & Zwi, J. D. (2004). The small world of the cerebral cortex. Neuroinformatics, 2(2), 145–162.
Tessitore, A., Esposito, F., Vitale, C., Santangelo, G., Amboni, M., Russo, A., Corbo, D., Cirillo, G., Barone, P., & Tedeschi, G. (2012). Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology, 79(23), 2226–2232.
Tinaz, S., Lauro, P., Hallett, M., & Horovitz, S. G. (2015). Deficits in task-set maintenance and execution networks in Parkinson’s disease. Brain Structure and Function, 220(1), 1–13.
Vaessen, M., Braakman, H., Heerink, J., Jansen, J., Debeij-van Hall, M., Hofman, P., Aldenkamp, A., & Backes, W. (2013). Abnormal modular organization of functional networks in cognitively impaired children with frontal lobe epilepsy. Cerebral Cortex, 23(8), 1997–2006.
Van Dijk, K. R., Sabuncu, M. R., & Buckner, R. L. (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59(1), 431–438.
van Eimeren, T., Monchi, O., Ballanger, B., & Strafella, A. P. (2009). Dysfunction of the default mode network in Parkinson disease: a functional magnetic resonance imaging study. Archives of Neurology, 66(7), 877–883.
Vincent, J. L., Kahn, I., Snyder, A. Z., Raichle, M. E., & Buckner, R. L. (2008). Evidence for a fronto-parietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100(6), 3328–3342.
Wang, J. H., Zuo, X. N., Gohel, S., Milham, M. P., Biswal, B. B., & He, Y. (2011). Graph theoretical analysis of functional brain networks: test-retest evaluation on short-and long-term resting-state functional MRI data. PloS One, 6(7), e21976.
Wu, T., & Hallett, M. (2013). The cerebellum in Parkinson’s disease. Brain, 136(3), 696–709.
Wu, T., Long, X., Wang, L., Hallett, M., Zang, Y., Li, K., & Chan, P. (2011b). Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Human Brain Mapping, 32(9), 1443–1457.
Wu, K., Taki, Y., Sato, K., Sassa, Y., Inoue, K., Goto, R., Okada, K., Kawashima, R., He, Y., & Evans, A. C. (2011a). The overlapping community structure of structural brain network in young healthy individuals. PloS One, 6(5), e19608.
Yan, C.-G., Cheung, B., Kelly, C., Colcombe, S., Craddock, R. C., Martino, A. D., Li, Q., Zuo, X.-N., Castellanos, F. X., & Milham, M. P. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage, 76(1), 183–201.
Zalesky, A., Fornito, A., Harding, I. H., Cocchi, L., Yücel, M., Pantelis, C., & Bullmore, E. T. (2010). Whole-brain anatomical networks: does the choice of nodes matter? NeuroImage, 50(3), 970–983.
Zuo, X.-N., Ehmke, R., Mennes, M., Imperati, D., Castellanos, F. X., Sporns, O., & Milham, M. P. (2012). Network centrality in the human functional connectome. Cerebral Cortex, 22(8), 1862–1875.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This study was funded by the National Natural Science Foundation of China (Grant numbers: 81271548, 81271560, 81371535, 81428013, and 81471654), and Zhejiang Provincial Natural Science Foundation of China (No. LZ13C090001).
Conflict of interest
All of the authors declare no conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Highlights
• Characterized altered modular organization in PD patients at early-to-mid clinical motor stages.
• PD patients showed increased intra-modular path length in mPFC, SN, FPN, and SMN.
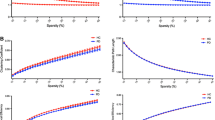
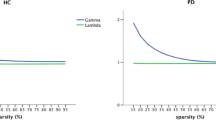
• Characteristic path length changed when confronted with ‘module lesion’
• Inter-modular functional connectivity between mPFC and SN can differentiate PD patients from controls.
Qing Ma and Biao Huang contributed equally to this work.
Electronic supplementary materials
ESM 1
(DOC 2.88 MB)
Rights and permissions
About this article
Cite this article
Ma, Q., Huang, B., Wang, J. et al. Altered modular organization of intrinsic brain functional networks in patients with Parkinson’s disease. Brain Imaging and Behavior 11, 430–443 (2017). https://doi.org/10.1007/s11682-016-9524-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9524-7