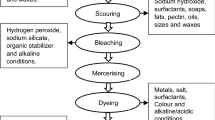
Abstract
Reports of environmental pollution by industries worldwide call for an urgent need to assess wastewater treatment facilities in various industries. This study presents an assessment of a wastewater treatment plant in an oral care (toothpaste) industry. The industry was visited, facilities for wastewater treatment were assessed (based only on efficacy to remove selected environmental and human’s health-related pollutants) and measurement of essential design parameters and facility characteristics were conducted. The study revealed that the averages of flow rate, biochemical oxygen demand at 5 days (BOD5), chemical oxygen demand (COD), suspended solids (SS), iron concentration, and total solids (TS) in the influent wastewater into the plant were 4.96 ± 0.6 m3/d; 90 ± 5 mg/L; 224 ± 8 mg/L; 1266.78 ± 10.24 mg/L; 0.31 ± 0.11 mg/L, and 2198.65 ± 20.44 mg/L, respectively. Individual efficacies were as follows: 0.49, 0.28, and 0.38% for SS, TS, and calcium, respectively. The overall efficacy of the wastewater treatment facility was found to be 0.020% which was significantly lower than expected. This indicates that no treatment was conducted on the wastewater and that the wastewater is being discharged into the environment essentially untreated. Equalization time (t eq) was found to be 2.0 h with equivalent equalized BOD5 concentration of 90 ± 5 mg/L, while expected volume for the equalization tank is 1.5 m3. It was concluded that failure (lower overall efficacy) of the system can be attributed to lack of an equalization tank, inadequate treatment processes, and refusal to apply standardized engineering code and practices. Although such conditions are rare in developed nations, these results demonstrate the problems in pollution control in developing communities.












Similar content being viewed by others
Abbreviations
- FEPA:
-
Federal Environmental Protection Agency
- GLUMRBSSE:
-
Great Lake Upper Mississippi River Board of State Sanitary Engineers
- MOP8:
-
Manual of Practice number 8
- ρ :
-
Mass density of the fluid, kg/m3 = ρ l
- θ s :
-
Angle of the rack with horizontal, °
- β s :
-
Bar shape factor
- μ s :
-
Dynamic viscosity of the fluid, N.s/m2
- Δt and t :
-
Time increment and time, respectively, d
- Δt eq :
-
Time interval over which samples were composite, h
- A sp :
-
Fine screen effective submerged open area, m2
- BOD5 :
-
Biochemical oxygen demand at 5 days, mg/L
- b s :
-
Minimum clear spacing of bars, m
- C 2eq :
-
Basin concentration after addition of flow for time Δt, mg/L
- C 2teq :
-
Basin concentration before addition of flow for time Δt, mg/L
- C ieq :
-
Basin average influent concentration over a period of Δt, mg/L
- COD:
-
Chemical oxygen demand, mg/L
- C si :
-
Fine screen coefficient of discharge, dimensionless
- D m :
-
Diameter of the impeller, m
- g :
-
Acceleration due to gravity, m/s2
- G :
-
Mean velocity gradient, m/s
- h Lc :
-
Head loss in a coarse screen, m
- h Lf :
-
Head loss in fine screens, m
- h v :
-
Velocity head of flow approaching the rack, m = \( k_{\text{v}} \left( {\frac{{V_{\text{a}}^{2} }}{2g}} \right) \)
- k mn :
-
Constant in mixing, varies with equipment used
- n r :
-
Number of revolution per second, rpm
- P l :
-
Power required in laminar condition, W
- P t :
-
Power required in turbulent condition, W
- P v :
-
Power required per unit volume, W/m3
- Q s :
-
Discharge through the fine screen, m3/d
- S eq :
-
Standard deviation of effluent wastewater concentration at a specified probability
- S iq :
-
Standard deviation of influent wastewater concentration
- t eq :
-
Equalization detention time, h
- w s :
-
Maximum cross-sectional width of the bars facing direction of flow, m
References
FEPA: Guidelines to Standards for Environmental Pollution Control in Nigeria. Federal Environmental Protection Agency (FEPA), Lagos (1991)
Ogedengbe, M.O., Ige, M.T., Ukatu, A.C.: The performance of a locally built mechanical system for grading filter sand. Int. J. Dev. Technol. 1, 189–197 (1983)
Babatola, J.O., Oladepo, K.T., Lukman, S., Olarinoye, N.O., Oke, I.: A failure analysis of a dissolved air flotation treatment plant in a dairy industry. J. Fail. Anal. Preven. 11(2), 110–122 (2011)
Nemerow, N.L.: Theories and Practices on Industrial Waste Treatment, 1st edn. Addison-Wesley, London (1963)
Fair, G.M., Geyer, J.C., Okun, D.A.: Elements of Water Supply and Wastewater Disposal, 2nd edn. Wiley, New York (1971)
Metcalf and Eddy, Inc.: Edited and Revised by Tchobanoglous, G, Burton, F. L. Wastewater Engineering, Treatment, Disposal and Reuse, 2nd edn. (1991)
Steel, E.W., McGhee, J.T.: Water Supply and Sewerage, 1st edn. McGraw-Hill, Tokyo (1979)
Noyes, R.: Unit Operations in Environmental Engineering, 1st edn. Noyes Publication, Park Ridge, NJ (1994)
Martins, J.E., Martins, T.E.: Technologies for Small Water and Wastewater Systems, 2nd edn. Van Nostrand Reinhold Company, New York (1993)
Eckenfelder, W.W.: Industrial Water Pollution Control, 2nd edn. McGraw-Hill, Tokyo (1989)
Humenick, M.J.: Water and Wastewater Treatment, Calculations for Chemical and Physical Processes, 1st edn. Marcel Dekker Inc, New York (1977)
MOP8: Sewage Treatment Plant Design, Water Pollution Control Federation and the America Society of Civil Engineers, Washington, DC (1967)
GLUMRBSSE: Recommended Standards for Sewage Work, 1968th edn. Health Education Service, New York (1968)
Oke, I.: A development and performance-testing of an electrochemical process for selected industrial wastewaters. Unpublished Ph.D. thesis, Civil Engineering Department, Obafemi Awolowo University, Ile-Ife (2007)
Adewuyi, G.O., Oputu, O.U., Opasina, M.A.: Assessment of groundwater quality and saline intrusions in coastal aquifers of Lagos Metropolis, Nigeria. J. Water Resour. Prot. 2, 849–853 (2010)
Acknowledgments
We wish to acknowledge Education Trust Fund (ETF) Nigeria and Obafemi Awolowo University, Ile-Ife, Nigeria for supporting this research work as part of preliminary studies to our research study titled “Electrochemical Treatment of Raw Water and Wastewaters.” Also, the authors wish to acknowledge Linkages Office of Obafemi Awolowo University, Ile-Ife, Nigeria for the Scientific Writing and Publishing & Oral Communication and Presentation Training given to the main author. We applied the sequence determines-credit (SDC) approach, which involves intellectual input, practical (data capture, data processing and organizing) input, specialist input, literary input and financial input for the sequence of the author.
Author information
Authors and Affiliations
Corresponding author
Appendix: Derivation of Equations for Treatment Plant’s Efficacy
Appendix: Derivation of Equations for Treatment Plant’s Efficacy
The pollutants that are removed by the treatment plant can be divided into two parts of a and b; “a” represents the turbidity removed and “b” represents microorganisms reduced specifically bacteria. Under normal condition or operation of wastewater treatment pollutants are expected to be removed or reduce to FEPA [1] standard limits. Let F a denote the concentration of pollutant in the raw water (F), O a denote the fraction of “a” removed from the raw wastewater but in the floc (O); and U a denote the fraction of “a” present in the treated wastewater (U). Then, the pollutant removed from the raw wastewater can be expressed as follows [2, 3]:
which can be expressed as follows [3]:
Elimination of O a or U a from the above equations
and
It can then be shown that the efficacies of the treatment plant in removing COD (E u) and BOD5 (E o) are as shown:
These indicate that overall efficiency of the treatment plant can be expressed as follows [2, 3]:
Rights and permissions
About this article
Cite this article
Oke, I.A., Oladepo, K.T., Olarinoye, N.O. et al. Failure Analysis of an Effluent Treatment Plant in an Oral Care Industry. J Fail. Anal. and Preven. 11, 565–577 (2011). https://doi.org/10.1007/s11668-011-9512-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-011-9512-6