Abstract
In oxidizing environments, most tin-based lead (Pb)-free alloys form a tin oxide that is easily eroded or mechanically damaged, affecting corrosion resistance and thus reliability of the soldered joints. In this study, the effect of microstructure heterogeneity on corrosion behavior of Pb-free solder candidate systems has been investigated on the example of as-cast and heat-treated alloys. The research was focused on a comparison between the corrosion resistance of binary Sn-Zn and ternary Sn-Zn-Cu alloys. Accelerated corrosion tests were performed by means of electrochemical methods in the sodium sulfate solution (VI), Na2SO4, of about 0.5 M concentration, pH adjusted to 2 by means of concentrated H2SO4 acid. In these tests, the corrosion potentials as well as polarization curves were determined for the selected alloys in as-cast state and after their heat treatment using different combinations of processing parameters. The measurements of basic electrochemical characteristics were made, i.e., the corrosion current (i corr μA/cm2) and Tafel coefficients, both cathodic (b c V/dec) and anodic (b a V/dec) ones. Detailed structural characterization of as-cast and heat-treated alloys before and after accelerated corrosion tests has been made under a wide range of magnifications using light microscopy and scanning electron microscopy observations. The results showed that structural heterogeneity of the examined alloys, attributed to the presence of secondary phases, and affected by their size and distribution, significantly influences the behavior of the examined Pb-free Sn-Zn-based alloys in the corrosive environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the considerable toxicity of lead (Pb), health concerns, and environmental as well as legislation reasons, efforts have been made to replace the conventionally used Sn-Pb solders by new Pb-free alloys. The Sn-Zn-based alloys belong to a family of promising Pb-free candidates to substitute the Sn-Pb eutectic solder alloy due to the low cost and melting temperature of 198 °C, which is close to that of the Sn-Pb eutectic solder (Ref 1) and by 20 °C lower than that of the Pb-free solders from SAC family (Ref 2). However, there are still some problems that should be solved for the Sn-Zn alloys, such as their poor oxidation resistance and embrittlement (Ref 3). In order to further enhance the technological and utility properties of Sn-Zn alloys, such as wettability, mechanical characteristics, oxidation, and creep resistance, an introduction of a small amount of alloying elements was proposed by many researchers. For example, the improvement in wetting properties by the reduction of surface tension of molten Sn-Zn alloy can be achieved by addition of Bi, while Nd, La, and In were reported to be effective in increasing the wetting area of Sn-Zn-Bi solders (Ref 4). Wang et al. (Ref 5) examined the effect of Ag, Al, and Ga on the wettability of Sn-9Zn-X solders for which the optimum additive amounts of 0.3, 0.005-0.02, and 0.5 mass% were indentified, respectively. In addition, rare earth elements, Cu, Bi, In can effectively improve the wettability, mechanical properties and oxidation resistance of eutectic Sn-Zn alloys (Ref 6, 7).
The effect of Al and Cr additions on the high-temperature oxidation resistance of Pb-free alloys from the Sn-Zn system was discussed in Ref 8.
In spite of numerous literature data on corrosion behavior, the influence of microstructure and processing parameters on the corrosion resistance of some Pb-free solder alloys and their joints has not been univocally defined. In oxidizing environments, most tin-based Pb-free alloys form a tin oxide that is easily eroded or mechanically damaged, affecting corrosion resistance and thus reliability of the soldered joints.
In this study, the effect of microstructure heterogeneity on corrosion behavior of Pb-free solder candidate systems has been investigated based on systematic studies of the correlation between structure and corrosion resistance of as-cast and heat-treated binary Sn-Zn and ternary Sn-Zn-Cu alloys.
Materials and Testing
Binary Sn-Zn alloys (SnZn4.5, SnZn9, SnZn13.5, wt.%) and ternary alloys of similar Zn content and 1 wt.% addition of Cu (SnZn4.5Cu1, SnZn9Cu1, and SnZn13.5Cu1) were produced from pure (99.9%) metals by melting in a graphite crucible under argon cover and next cast into a graphite die. Cast alloy samples were heat treated using different combinations of the following processing parameters (Fig. 1): 168 h/50 °C, 42 h/80 °C, and 24 h/110 °C.
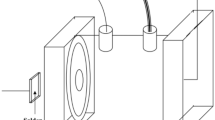
The accelerated corrosion tests were used to measure such electrochemical parameters as the corrosion current (i corr μA/cm2) and Tafel coefficients, both cathodic (b c V/dec) and anodic (b a V/dec) ones. These tests were performed by means of electrochemical method (polarization experiments) in the sodium sulfate solution (VI), Na2SO4, of about 0.5 M concentration, pH adjusted to 2 using concentrated H2SO4 acid.
Polarization experiments were carried out with a scan rate of 1 mV/s in the range of −100 to 100 mV versus open circuit potentials. Prior to each polarization experiment, the samples were immersed into electrolyte for 1 h while monitoring the open circuit potential to establish steady state conditions. A three-electrode cell arrangement was used, with a silver-silver chloride electrode (Ag/AgCl) as reference electrode and a platinum wire as the auxiliary electrode (counter electrode). The measurements were carried out by means of Autolab EcoChemie System of AUTOLABPGSTAT 302N type using GPESv.4.9 software for acquisition and processing of experimental data. The corrosion potential characteristics as well as polarization curves were determined for all the selected alloys, both as-cast and heat treated.
Structural characterization of freshly polished samples before and after accelerated corrosion tests was made by means of light microscopy (LM) using phase contrast technique and scanning electron microscopy (SEM) coupled with EDS analysis.
Results and Discussion
The results of accelerated corrosion tests made for reference Sn-Zn alloys (Fig. 2) and summarized results of the measurements of selected electrochemical parameters for both Sn-Zn and Sn-Zn-Cu alloys (Table 1) show a significant influence of the alloy composition on the corrosion resistance of as-cast solder candidates.
Among examined alloys, the SnZn13.5 alloy demonstrates the worst corrosion resistance since its i corr value is about 40 times higher when compared to that of the other alloys. For binary alloys, the SnZn4.5 and the SnZn9 samples are characterized by higher values of the anodic Tafel coefficient (b a), while the SnZn13.5 alloy has higher values of the cathodic Tafel coefficient (b c). The results collected in Table 1 and Fig. 2 suggest that at lower Zn contents, the dissolution of Sn matrix is preferred, while the deposition of corrosion products takes place at high Zn contents.
Significant improvement in corrosion resistance of selected as-cast Sn-Zn alloys was noted after introduction of 1 wt.% Cu. However, the increase in Zn content in the Sn-Zn-Cu alloys results in the reduction of their corrosion resistance (decrease in i corr values), while the values of corrosion current (i corr) as well as Tafel coefficients, both cathodic (b c) and anodic (b a), become comparable. These measurements suggest that the dissolution as well as corrosion products deposition in the examined Sn-Zn-Cu alloys take place with the similar intensity.
The corrosion potentials of binary and ternary alloys tend to become more negative with increasing Zn content but the differences between E corr values are smaller in ternary alloys than in the binary ones.
Structural observations carried out on freshly polished samples before and after corrosion accelerated tests using LM and SEM techniques evidenced that low corrosion resistance of as-cast SnZn13.5 alloy is caused by the presence of relatively coarse needle-like Zn-rich precipitates distributed in β-Sn matrix (Fig. 3a). On the contrary, only small quantities of much smaller Zn-rich phase precipitates were identified in as-cast Sn-Zn-Cu alloys.
This beneficial effect on the size, morphology, and number of Zn-rich precipitates is attributed to alloying with 1 wt.% Cu since Zn is partially consumed for the formation of probably Cu5Zn8 intermetallic compound (Fig. 3b to d). As expected, this more favorable microstructure of the examined Sn-Zn-Cu alloys contributes to their better corrosion resistance, compared to binary Sn-Zn alloys with similar Zn content.
The results of accelerated corrosion tests carried out on heat-treated Sn-Zn alloys are presented in Table 2 and in Fig. 4, showing the best corrosion resistance for SnZn4.5 alloy after the 42 h/80 °C treatment and results comparable in the case of SnZn4.5 and SnZn9 alloys after the 24 h/110 °C treatment.
Similar to as-cast alloys, the lowest corrosion resistance after the heat treatment was identified for binary SnZn13.5 alloy. Although the heat treatment significantly improved the corrosion resistance of all the examined binary Sn-Zn alloys, this tendency is different and depends on the alloy chemical composition.
Detailed structural characterization of heat-treated alloys after corrosion tests has evidenced that also in that case, similar to as-cast alloys, the lowest corrosion resistance occurs in SnZn13.5 alloy due to the presence of coarse needle-like Zn-rich phase embedded in β-Sn matrix (Fig. 5). However, since these precipitates in the heat-treated alloy are much smaller than those in as-cast alloy (Fig. 3a), the value of corrosion current becomes two times lower after heat treatment.
For heat-treated Sn-Zn-Cu alloys (Table 3; Fig. 6), the best results were obtained with SnZn4.5Cu1 alloy for 168 h/50 °C conditions. Similar results of measurements were also recorded in the same alloy but subjected to a different selected regime of heat treatment. This positive effect of heat treatment on corrosion resistance improvement is mainly related with the absence of needle-like Zn-rich precipitates in the SnZn4.5Cu1 alloy (Fig. 6). On the contrary, the as-cast SnZn9Cu1 and SnZn13.5Cu1 alloys (Table 1) show better corrosion resistance than the heat-treated ones. In these alloys, both needle-like Zn-rich and probably Cu5Zn8 precipitates may exist in β-Sn matrix but heat treatment causes an increase in their number and size (Fig. 7). Therefore, higher Zn content causes worse corrosion resistance, independent of the heat treatment parameters.
Conclusions
The results indicate the crucial role of the alloy microstructure, particularly the presence of secondary phases, on corrosion behavior of Pb-free solder candidates from both binary Sn-Zn and ternary Sn-Zn-Cu systems as examined by accelerated corrosion tests in the sodium sulfate solution (VI), Na2SO4, of about 0.5 M concentration, pH adjusted to 2 by means of concentrated H2SO4 acid.
In particular, the control of Zn-rich phase precipitation in β-Sn matrix is the most important aspect among possible solutions for an improvement of the corrosion resistance of Pb-free solder candidates from selected systems. For binary Sn-Zn alloys of lower Zn content, this may be achieved by the appropriate selection of processing parameters and/or by alloying with Cu, when probably Cu5Zn8 precipitates are formed instead of large Zn-rich crystals. An increase in Zn content deteriorates the corrosion resistance of both Sn-Zn and Sn-Zn-Cu as-cast alloys, and it becomes dramatically low when the alloy contains 13.5 wt.% Zn.
For all the alloys, the best corrosion resistance was recorded after their heat treatment, particularly for the following processing parameters:
-
SnZn4.5 alloy—42 h/80 °C and 24 h/110 °C because of the same grain size and the lack of Zn-rich phase precipitates,
-
SnZn9 alloy—24 h/110 °C because of smaller quantities of Zn-rich phase precipitates, contrary to longer time processing,
-
SnZn4.5Cu1 alloy—the satisfactory effects for all the selected combinations of processing parameters since small values of corrosion currents and slight differences between them were recorded due to beneficial change in the alloy microstructure caused from the formation of probably Cu5Zn8 precipitates instead of Zn-rich precipitates,
-
SnZn9Cu1 alloy—independent of processing parameters, a negative effect of Zn content was observed because of the presence of Zn-rich precipitates, but it was the smallest for 42 h/80 °C heat treatment,
-
SnZn13.5Cu1 alloy—the negative effect of Zn content was also identified while the biggest Zn-rich precipitates were formed during heat treatment at the highest temperature (24 h/110 °C).
Moreover, for the Sn-Zn-Cu alloys of higher Zn content (9% and 13.5%), the presence of 1% Cu did not improve their corrosion behavior.
References
Y. Min, L. Xiuzhong, L. Xinghong, and D. Jiahui, Development of Sn-Zn-Cu Lead Free Solder, Conference Proceedings of 11th International Conference ICEPT-HDP, 2010, p 784–788
K.L. Lin and C.L. Shih, Microstructure and Thermal Behavior of Sn-Zn-Ag Solders, J. Electron. Mater., 2003, 32(12), p 1496–1500
K. Suganuma and K.S. Kim, Sn-Zn Low Temperature Solder, J. Mater. Sci. Mater. Electron., 2007, 18(1–3), p 121–127
J. Zhou, Y.S. Sun, and F. Xue, Effect of Nd and La on Surface Tension and Wettability of Sn-8Zn-3Bi, Trans. Nonferrous Met. Soc. China, 2005, 15(5), p 1161–1165
H. Wang, S.B. Xue, W.X. Chen, and J.X. Wang, Effect of Ag, Al, Ga Addition on Wettability of Sn-9Zn Lead-Free Solder, Trans. China Weld. Inst., 2007, 28(8), p 33–36
H. Wang, S.B. Xue, Z.J. Han, and J.X. Wang, Research Status and Prospect of Sn-Zn Based Lead-Free Solders, Weld. J., 2007, 2, p 31–35
S. Wu, H. Kang, and P. Qu, Study of Sn-Zn Lead-Free Solder by Alloying, Electron. Process Technol., 2008, 29(2), p 66–70
X.X. Ren, M. Li, and D.L. Mao, Effect of Alloying Elements on the High-Temperature Oxidation Resistance of Sn-Zn Based Lead-Free Solder, Electron. Compon. Mater., 2004, 23(11), p 40–44
Acknowledgments
The research was carried out within the framework of the EU COST ACTION MP0602 project with the financial support from the Ministry of Science and Higher Education of Poland.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited submission to JMEP selected from presentations at the Symposia “Wetting, soldering and brazing” and “Diffusion bonding and characterization” belonging to the Topic “Joining” at the European Congress and Exhibition on Advanced Materials and Processes (EUROMAT 2011), held September 12-15, 2011, in Montpellier, France, and has been expanded from the original presentation.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pietrzak, K., Grobelny, M., Makowska, K. et al. Structural Aspects of the Behavior of Lead-Free Solder in the Corrosive Solution. J. of Materi Eng and Perform 21, 648–654 (2012). https://doi.org/10.1007/s11665-012-0145-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-012-0145-z