Abstract
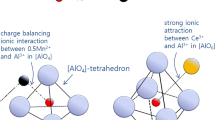
Sulfide capacity of the MnO-SiO2-Al2O3-Ce2O3 system was measured at 1873 K (1600 °C), and the structural analysis was carried out using micro-Raman spectroscopy to understand the role of Ce2O3 in the sulfur dissolution behavior. Sulfide capacity of the basic melts (MnO/SiO2 = 2.2(±0.14)) decreased with increasing content of Ce2O3 to approx. 4 mol pct, beyond which it increased. Sulfide capacity continuously decreased in the less basic system (MnO/SiO2 = 1.0(±0.15)), whereas it was hardly affected by Ce2O3 in the relatively acidic composition (MnO/SiO2 = 0.3(±0.05)). There was a significant increase in the intensity of Raman band at 600 cm−1 by Ce2O3 addition in high MnO/SiO2 (=2.2) system, which originated from the transition from [(Al,Mn0.5)O4]-tetrahedron to [(Al,Ce)O6]-octahedron due to strong attraction between Al2O3 and Ce2O3. Combining thermodynamic and structural information, the effect of Ce2O3 on the sulfide capacity of Mn-aluminosilicate melts can be explained by the following factors: (1) Activity of MnO in the melts decreased by addition of Ce2O3; (2) Free oxygen was consumed in the structure modification from [(Al,Mn0.5)O4] to [(Al,Ce)O6] unit by addition of Ce2O3; and 3) When the Ce3+ content was greater than critical value (approx. 4 mol pct) in high MnO/SiO2 (=2.54) melts, excess Ce3+ and Mn2+ ions competitively reacted with S2− ions, resulting in an increase of sulfide capacity.











Similar content being viewed by others
References
S. T. Kim, S. H. Jeon, I. S. Lee and Y. S. Park: Corros. Sci., 2010, vol. 52, pp. 1897-1904.
S. H. Jeon, S. T. Kim, I. S. Lee and Y. S. Park: Corros. Sci., 2010, vol. 52, pp. 3537-47.
S. K. Kwon, Y. M. Kong and J. H. Park: Met. Mater. Int., 2014, vol. 20, pp. 959-66.
S. K. Kwon, J. S. Park and J. H. Park: ISIJ Int., 2015, vol. 55, pp. 2589-96.
R. A. Sharma and F. D. Richardson: Trans. Metall. Soc. AIME, 1965, vol. 233, pp. 1586-92.
M. M. Nzotta: Scand. J. Metall., 1997, vol. 26, pp. 169-77.
Y. B. Kang and A. D. Pelton: Metall. Mater. Trans. B, 2009, vol. 40B, pp. 979-94.
D. H. Woo, Y. B. Kang and H. G. Lee: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 915-20.
H. Ohta and H. Suito: Metall. Mater. Trans. B, 1996, vol. 27B, pp. 263-70.
T. Fujisawa and H. Sakao: Tetsu-to-Hagane, 1977, vol. 63, pp. 1504-11.
N. M. Anacleto, H. G. Lee and P. C. Hayes: ISIJ Int., 1993, vol. 33, pp. 549-55.
G. H. Park, Y. B. Kang and J. H. Park: ISIJ Int., 2011, vol. 51, pp. 1375-82.
J. H. Park and G. H. Park: ISIJ Int., 2012, vol. 52, pp. 764-69.
J. H. Park: Steel Res. Int., 2013, vol. 84, pp. 664-69.
J. H. Park: J. Non-Cryst. Solids, 2012, vol. 358, pp. 3096-3102.
J. H. Park, K. Y. Ko and T. S. Kim: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 741-48.
J. H. Park: ISIJ Int., 2012, vol. 52, pp. 1627-36.
E. J. Preisler, O. J. Marsh, R. A. Beach and T. C. McGill: J. Vac. Sci. Technol. B, 2001, vol. 19, pp. 1611-18.
J.A-Rivera, D.A.R. Carvajal, M. del C. A-Enriquez, M.B.M-Martinez, E. Alvarez, R. L-Morales, G. C. Diaz, A. de Leon, and M. E. Zayas: J. Am. Ceram. Soc., 2014, vol. 97, pp. 3494–500.
M. Sekita, A. Fujimori, A. Makishima, T. Shimohira: J. Non-Cryst. Solids, 1985, vol. 76, pp. 399-407.
A. Makishima, M. Kobayashi, and T. Shimohira: Commun. Am. Ceram. Soc., 1982, vol. 65, C-210-C-211.
C. J. B. Finchan and F. D. Richardson: Proc. Roy. Soc. London A, 1954, vol. 223, pp. 40-62.
R. G. Ward: An Introduction to the Physical Chemistry of Iron and Steelmaking, Edward Arnold, 1962.
Y. B. Kang and J. H. Park: Metall. Mater. Trans. B, 2011, vol. 42B, pp. 1211-17.
A. Vahed and D. A. R. Kay: Metall. Trans. B, 1976, vol. 7B, pp. 375-83.
S. L. Lin, C. S. Hwang and J. F. Lee: Jpn. J. Appl. Phys., 1996, vol. 35, pp. 3975-83.
P. Wu and A. D. Pelton: J. Alloy. Compd., 1992, vol. 179, pp. 259-87.
S. Ueda, K. Morita and N. Sano: ISIJ Int., 1998, vol. 38, pp. 1292-96.
R. Kitano, M. Ishii and K. Morita: Proc. of 6 th Asia Steel Int. Conf., 2015, pp. 40-41.
J. H. Park, D. J. Min and H. S. Song: Metall. Mater. Trans. B, 2004, vol. 35B, 269-275.
B. O. Mysen, D. Virgo and C. M. Scarfe: Am. Mineral., 1980, vol. 65, pp. 690-710.
J. H. Park: Met. Mater. Int., 2013, vol. 19, pp. 557-84.
K. Chah, B. Boizot, B. Reynard, D. Ghaleb and G. Petite: Nucl. Instrum. Meth. B, 2002, vol. 191, pp. 337-41.
F. Seifert, B. O. Mysen and D. Virgo: Am. Mineral., 1982, vol. 67, pp. 696-718.
P. McMillan, B. Piriou and A. Navrotsky: Geochim.Cosmochim. Acta, 1982, vol. 46, pp. 2021-37.
M. Okuno, N. Zotov, M. Schmucker and H. Schneider: J. Non-Cryst. Solids, 2005, vol. 351, pp. 1032-38.
T.S. Kim and J.H. Park: ISIJ Int., 2014, vol. 54, pp. 2031-38.
J.H. Park, D.J. Min and H.S. Song: ISIJ Int., 2002, vol. 42, pp. 38-43.
B. O. Mysen and P. Richet: Silicate Glasses and Melts: Properties and Structure, Elsevier, Amsterdam, Netherlands, 2005.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant Number NRF-2012R1A1A2041774). Furthermore, the authors express the appreciation to Professor KAZUKI MORITA, The University of Tokyo, Japan, for a fruitful discussion in regard of the interaction between Ce2O3 and Al2O3 in oxide melts.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted July 28, 2016.
Rights and permissions
About this article
Cite this article
Jeong, S.J., Kim, T.S. & Park, J.H. Relationship Between Sulfide Capacity and Structure of MnO-SiO2-Al2O3-Ce2O3 System. Metall Mater Trans B 48, 545–553 (2017). https://doi.org/10.1007/s11663-016-0828-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0828-1