Abstract
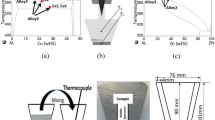
The novelty of the controlled diffusion solidification (CDS) process is the mixing of two precursor alloys with different thermal masses to obtain the resultant desired alloy, which is subsequently cast into a near-net-shaped product. The critical event in the CDS process is the ability to generate a favorable environment during the mixing of the two precursor alloys to enable a well-distributed and copious nucleation event of the primary Al phase leading to a nondendritic morphology in the cast part. The turbulence dissipation energy coupled with the undercooling of the precursor alloy with the higher temperature enables the copious nucleation events, which are well distributed in the resultant mixture.










Similar content being viewed by others
Notes
PANDAT 8.1 is a trademark of CompuThermLLC, Madison, WI.
SCXI-1100 is a trademark of National Instruments, Vaudreuil-Dorion, PQ, Canada.
NIKON AZ 100M is a trademark of Eberbach Corporation, Ann Arbor, MI.
JEOL is a trademark of Japan Electron Optics Ltd., Tokyo.
Abbreviations
- Alloy 1:
-
precursor alloy with higher thermal mass (higher temperature and higher mass)
- Alloy 2:
-
precursor alloy with lower thermal mass
- Alloy 3:
-
resultant desired alloy
- C1 and C2:
-
average solute concentration of Alloys 1 and 2, respectively
- C Cu :
-
transient solute concentration (Cu)
- f :
-
final
- L :
-
characteristic length
- m1, m2, and m T :
-
mass of Alloy 1, Alloy 2, and total mass, respectively
- NWe :
-
Weber number
- S/L:
-
solid-liquid interface
- T1 and T2:
-
temperatures of Alloys 1 and 2, respectively
- T actual :
-
actual temperature
- T B :
-
temperature of point B
- TL1, TL2, TL3:
-
liquidus temperatures of Alloys 1, 2, and 3, respectively
- T m :
-
melting temperature
- T quench :
-
quenching temperature
- u :
-
velocity
- ξ :
-
transient instantaneous position
- ρ :
-
density
- μ :
-
dynamic viscosity
- σ :
-
surface tension
References
W. Kurz and D.J. Fisher: Fundamentals of Solidification, 3rd ed., Trans Tech Publications, Aedermannsdorf, Switzerland, 1989, pp. 133–53.
G. Wang, P. Lu, H.Y. Wang, and Q.C. Jiang: Mater. Lett., 2004, vol. 58, pp. 3852–56.
Q.Q. Zhang, Z.Y. Cao, Y.F. Zhang, G.H. Su, and Y.B. Liu: J. Mater. Process. Technol., 2007, vol. 184, pp. 195–200.
H.Q. Lin, J.G. Wang, H.Y. Wang, and Q.C. Jiang: J. Alloys Compd., 2007, vol. 431, pp. 141–47.
E.J. Zoqui: J. Mater. Process. Technol., 2003, vols. 143–144, pp. 195–201.
P.K. Seoa, S.M. Leeb, and C.G. Kang: J. Mater. Process. Technol., 2009, vol. 209, pp. 171–80.
G. Hongmin1, Y. Xiangjie, and H. Bin: J. Wuhan Univ. Technol. Mater. Sci., 2008, Feb.
M. Rappaz, J.M. Drezet, and M. Gremaud: Metall. Mater. Trans. A, 1999, vol. 30A, pp. 449–55.
D. Saha, S. Shankar, D. Apelian, and M.M. Makhlouf: Metall. Mater. Trans. A, 2004, vol. 35A, pp. 2174–80.
K. Symeonidis: Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, Apr. 2009, pp. 44–49.
D. Apelian, M.M. Makhlouf, and D. Saha: Mater. Sci. Forum, 2006, vols. 519–521, pp. 1771–76.
D. Saha, S. Shankar, D. Apelian, and M. Makhlouf: Proc. John Campbell Honorary Symp., P. Crepeau and M. Tiryakioglu, eds., TMS, Warrendale, PA, 2004.
W.T. Olsen and R. Hultgren: Trans. AIME, 1950, vol. 188, p. 1323.
P. Duwez, R.H. Willens, and W. Klement, Jr.: J. Appl. Phys., 1960, vol. 31, p. 1136.
V. Vishan and A.V. Narlikar: Mater. Res. Bull., 1976, vol. II, pp. I257–64.
A.A. Khalaf, P. Ashtari, and S. Shankar: Shaped Casting: The 3rd Int. Symp., J. Campbell, P.N. Crepeau, and M. Tiryakioglu, eds., TMS, Warrendale, PA, 2009, pp. 215–22.
A.A. Khalaf, P. Ashtari, and S. Shankar: Metall. Mater. Trans. B, 2009, vol. 40B, pp. 843–49.
A.A. Khalaf: Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 2010, pp. 72–90.
H. Bruus: Theoretical Microfluidics, Oxford University Press, Oxford, United Kingdom, 2008, pp. 123–36.
P. Srirangam: Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, Apr. 2010.
A. Wierzba: Exp. Fluids, 1990, vol. 9, pp. 59–64.
S.M. Oak, B.J. Kim, W.T. Kim, M.S. Chun, and Y.H. Moon: J. Mater. Process. Technol., 2002, vols. 130–131, pp. 304–09.
M.C. Flemings: Solidification Processing, McGraw-Hill, New York, NY, 1974, pp. 32–57.
D. Apelian and M.M. Makhlouf: High Integrity Aluminum Die Casting (Alloys, Processes and Melt Preparation), North American Die Casting Association, Rosemont, IL, 2006, Item 307, pp. 3–15.
Acknowledgments
The authors acknowledge the financial support provided by the Natural Science and Engineering Research Council (NSERC) of Canada through their Discovery Grant Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted October 30, 2010.
Rights and permissions
About this article
Cite this article
Khalaf, A.A., Shankar, S. Favorable Environment for a Nondendritic Morphology in Controlled Diffusion Solidification. Metall Mater Trans A 42, 2456–2465 (2011). https://doi.org/10.1007/s11661-011-0641-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-011-0641-z