Abstract
Background
Emergency treatment of bleeding esophageal varices in cirrhosis is of singular importance because of the high mortality rate. Emergency portacaval shunt is rarely used today because of the belief, unsubstantiated by long-term randomized trials, that it causes frequent portal-systemic encephalopathy and liver failure. Consequently, portacaval shunt has been relegated solely to salvage therapy when endoscopic and pharmacologic therapies have failed. Question: Is the regimen of endoscopic sclerotherapy with rescue portacaval shunt for failure to control bleeding varices superior to emergency portacaval shunt? A unique opportunity to answer this question was provided by a randomized controlled trial of endoscopic sclerotherapy versus emergency portacaval shunt conducted from 1988 to 2005.
Methods
Unselected consecutive cirrhotic patients with acute bleeding esophageal varices were randomized to endoscopic sclerotherapy (n = 106) or emergency portacaval shunt (n = 105). Diagnostic workup was completed and treatment was initiated within 8 h. Failure of endoscopic sclerotherapy was defined by strict criteria and treated by rescue portacaval shunt (n = 50) whenever possible. Ninety-six percent of patients had more than 10 years of follow-up or until death.
Results
Comparison of emergency portacaval shunt and endoscopic sclerotherapy followed by rescue portacaval shunt showed the following differences in measurements of outcomes: (1) survival after 5 years (72% versus 22%), 10 years (46% versus 16%), and 15 years (46% versus 0%); (2) median post-shunt survival (6.18 versus 1.99 years); (3) mean requirements of packed red blood cell units (17.85 versus 27.80); (4) incidence of recurrent portal-systemic encephalopathy (15% versus 43%); (5) 5-year change in Child’s class showing improvement (59% versus 19%) or worsening (8% versus 44%); (6) mean quality of life points in which lower is better (13.89 versus 27.89); and (7) mean cost of care per year ($39,200 versus $216,700). These differences were highly significant in favor of emergency portacaval shunt (all p < 0.001).
Conclusions
Emergency portacaval shunt was strikingly superior to endoscopic sclerotherapy as well as to the combination of endoscopic sclerotherapy and rescue portacaval shunt in regard to all outcome measures, specifically bleeding control, survival, incidence of portal-systemic encephalopathy, improvement in liver function, quality of life, and cost of care. These results strongly support the use of emergency portacaval shunt as the first line of emergency treatment of bleeding esophageal varices in cirrhosis.
Similar content being viewed by others
Introduction
Emergency treatment of bleeding esophageal varices (BEV) in patients with cirrhosis of the liver is of singular importance because of the high mortality rate surrounding the episode of acute bleeding.1–9 The most widely used emergency treatment of BEV is endoscopic sclerotherapy (EST) or endoscopic variceal ligation (EVL), with or without the addition of pharmacologic measures.10–12 When it is believed that portal decompression is needed, transjugular intrahepatic portosystemic shunt (TIPS) has become the most widely used procedure of choice despite the facts that, as we have pointed out previously, TIPS has a high rate of stenosis and occlusion, a resultant high incidence of portal-systemic encephalopathy (PSE), and limited durability. TIPS occlusion rate has been reduced by the recent introduction of the polytetrafluorethylene-coated stent, but the rates of occlusion and PSE are still much higher than the incidences of these serious complications following portacaval shunt in all of our studies.
Emergency portacaval shunt (EPCS) is rarely used today because of the belief, unsubstantiated by randomized controlled trials involving unselected patients, that EPCS causes frequent portal-systemic encephalopathy and liver failure.4,13–21 Consequently, portacaval shunt (PCS) has been relegated solely to the salvage of failed endoscopic and pharmacologic treatment. An important question is: is the regimen of EST or ligation with rescue PCS for failure to control BEV superior to EPCS? A unique opportunity to compare the regimen of EST with rescue PCS with EPCS was provided by our randomized controlled trial (RCT) of EST versus EPCS known as the San Diego Bleeding Esophageal Varices Study.
From April 8, 1988 to December 31, 2005, we conducted a RCT in 211 unselected, consecutive patients with cirrhosis and acute BEV in whom emergency and long-term EST was compared with direct EPCS, otherwise known as total shunt. The trial was a community-wide endeavor that involved patients referred from four adjacent counties to the University of California, San Diego (UCSD) Medical Center. In two recent publications, we described the study in detail and reported the outcomes first with regard to control of bleeding and survival22 and second with regard to the development of PSE.23 This report focuses on a comparison of outcomes following the regimen of EST with rescue PCS to outcomes following EPCS.
Patients and Methods
The reader is directed to our two recent publications22,23 that provide detailed descriptions of the following methods and protocols used in this RCT:
- 1.
-
2.
Patient eligibility
-
3.
Definitions of:
-
(a)
Bleeding esophageal varices
-
(b)
Unselected patients (all comers)
-
(c)
Emergency EST
-
(d)
Long-term EST
-
(e)
Emergency portacaval shunt
-
(f)
Failure of emergency primary therapy
-
(g)
Failure of long-term therapy
-
(h)
Rescue therapy
-
(i)
Informed consent
-
(a)
-
4.
Randomization
-
5.
Diagnostic workup26
- 6.
-
7.
Initial emergency therapy during workup
-
8.
Endoscopic sclerotherapy
-
9.
Emergency portacaval shunt29
-
10.
Lifelong follow-up
-
11.
Quantitation of PSE
In addition, the RCT involved the following protocols that have not been described previously.
Rescue Portacaval Shunt
Rescue PCS was performed in 50 patients as soon as possible after failure of EST was declared. Direct side-to-side PCS was done in 46 patients (92%), and direct end-to-side PCS was done in four patients (8%). Operative technique and intraoperative pressure measurements were identical to those used in EPCS.
QOL Score
Quality of life (QOL) was measured by assessing the following factors: (1) liver function as determined by quantitative Child’s risk class; (2) development of recurrent PSE; (3) number of PSE episodes; (4) units of packed red blood cell (PRBC) transfusion for upper gastrointestinal bleeding; (5) number of hospital readmissions; (6) days of hospitalization during readmission; (7) return to work, including housekeeping; (8) abstinence from alcoholism; and (9) portacaval shunt patency. These nine factors were weighted numerically so as to produce a QOL score in which the lower the score, the better the QOL.
Direct Cost of Care
All hospital and outpatient facility charges and all professional fee bills from UCSD and from referring hospitals and physicians were obtained continuously for every patient entered into the study for 10 years.
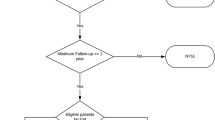
Figure 1 is a Consort flow diagram that shows the overall design and conduct of the RCT.22,23
Statistical Analysis
The comparison between Emergency and Rescue PCS groups used Fisher’s exact test for binary outcomes (e.g., control of bleeding, incidence of recurrent PSE) and Wilcoxon rank-sum test (WRT) for continuous outcomes (e.g., units of PRBC transfusion, number of recurrent PSE episodes, number of hospital readmissions). The length of survival was compared using Gehan–Wilcoxon rank test. The change in Child’s class was compared for each time interval using the exact WRT, adjusted for ties. The average change in Child’s class during the first 5 years was computed by averaging the duration of time in years spent by the patients at risk (alive) in each category (improved, unchanged, or worse). The comparison of the cause of recurrent PSE episodes used Pearson’s chi-squared test. The overall quality of life score was computed for each group and each year by adding up the scores of the nine components. This score was compared between the two groups assuming a Poisson (log-linear) model, with different means for the different categories, and a constant group effect. At the beginning of the study, it was decided in advance not to perform an interim analysis of the data.
Results
EPCS Versus EST—Outcome Data
Our recent publications should be consulted for detailed data on the clinical characteristics of the 211 patients, findings on upper endoscopy and liver biopsy, results of laboratory blood tests, data on rapidity of therapy, data on control of bleeding, operative and endoscopic data, data on PSE, and data on survival.22,23 There were no significant differences in the clinical characteristics of the two groups on entry in the RCT. Cirrhosis was demonstrated by liver biopsy in all patients. Definitive treatment was initiated in <24 h after onset of bleeding in all patients. EPCS controlled bleeding permanently in all patients, while EST achieved permanent control of bleeding in only 20%. Survival rates were significantly higher after EPCS than after EST at all time intervals and in all Child’s classes (p < 0.001). Patients with the most severe liver disease in Child’s risk class C realized substantial long-term survival after EPCS.
The incidence of recurrent PSE following EST was 35%, which was more than twice the 15% incidence following EPCS (p < 0.001). EST patients had a total of 179 episodes of PSE and 146 PSE-related hospital admissions compared with EPCS patients who had 94 episodes of PSE and 87 hospital admissions (p = 0.003). Recurrent UGI bleeding, which was rare in the EPCS group, was a major causative factor of PSE in the EST patients.
EST with Rescue PCS Versus EPCS
Patient Characteristics
Table 1 summarizes the clinical characteristics at the time of entry in the San Diego BEV study of the 105 patients who were randomized to EPCS and the 50 patients who failed EST and underwent rescue PCS. There were no significant differences between the two groups in any important characteristics of cirrhosis and BEV. Thirty-one patients failed EST but did not undergo rescue PCS for various reasons, most prominent of which were death from recurrent BEV at home or at a distant hospital and death from massive recurrence of BEV before a rescue PCS could be done. As others have found, failure of patients to take advantage of rescue treatment reflects the realities of treating BEV in the cirrhotic population. Although these 31 patients were excluded from the analysis, their deaths have a negative impact on the concept of rescue PCS for failed EST.
Table 2 summarizes data on rapidity of therapy and indicates clearly that all patients underwent rapid diagnosis and treatment upon entry in the RCT. Median time from onset of bleeding to the start of therapy was <24 h in both groups of patients. The time from initial contact at UCSD Medical Center to start of therapy was <8 h in every patients in the EST group and in 102 of the 105 patients in the EPCS group. Active bleeding was observed within 4 h of entry in the study in 83% of the 155 patients.
Control of Bleeding
Table 3 provides data on control of BEV by EPCS and by EST with rescue PCS. EPCS promptly and permanently controlled bleeding in every patient. In contrast, EST failed to control bleeding in any of the 50 patients, and that is why they underwent rescue PCS. Failure of EST in 106 patients in the EST group was based on one or more of the criteria established in advance by the study protocol, which included: (1) in 15 patients, variceal bleeding continued or recurred during the first 7 days after initial EST and required ≥6 U blood transfusion; (2) in 47 patients, recurrent variceal bleeding required ≥8 U of blood transfusion during any 12-month period after the index hospitalization; (3) in 27 patients, variceal bleeding recurred after an experienced co-investigator faculty gastroenterologist had previously declared that the esophageal varices were obliterated or gone. In eight of these same patients, recurrent bleeding required ≥8 U of blood transfusion, so they met two criteria of failure.
Table 3 also summarizes the requirement for PRBC transfusions. Overall, patients treated by EST with rescue PCS required almost twice the number of PRBC transfusions as patients treated by primary EPCS (p < 0.001).
Survival
Table 4 shows data on survival in the two groups of patients, and Fig. 2 shows 15-year Kaplan–Meier estimated survival plots. All patients in the EST-rescue PCS group and 98 of the 105 patients in the EPCS group were eligible for ten or more years of follow-up. The remaining seven EPCS patients had follow-up for 9.4–9.9 years. No patients were lost to follow-up. After the first year, there were highly significant differences in the survival rates of the two study groups at all long-term time intervals. The 5-, 10-, and 15-year survival rates in the EST-rescue PCS group were 22%, 16%, and 0%, respectively, and in the EPCS group were 72%, 46%, and 46%, respectively (p < 0.001). Median survival was 6.15 years in patients randomized to EPCS compared to 3.1 years in EST-rescue PCS patients (p < 0.001). Hepatic failure was the primary cause of death in 44% of patients who underwent EST with rescue PCS compared to 22% of patients who received primary EPCS. In contrast to the entire group of 106 EST patients in which 26% died from variceal bleeding, none of the 105 EPCS patients died of bleeding.
As expected, the survival rate was related to the severity of liver disease at the time of entry in the study, as expressed by quantitative Child’s risk classes. In the EST group with rescue PCS, 5-year survival rates in Child’s classes A, B, and C were 36%, 15%, and 20%, respectively, and 10-year survival rates in Child’s classes A, B, and C were 29%, 12%, and 10%, respectively. In contrast, in the EPCS group, the corresponding survival rates in Child’s classes A, B, and C were 89%, 76%, and 53% at 5 years and 62%, 47%, and 30% at 10 years. The differences in favor of EPCS were highly significant (p = 0.005 to p < 0.001).
Median survival of patients who failed EST and underwent a rescue PCS was 3.01 years compared to median survival of 2.36 years in the 38% of patients who failed EST but did not undergo a rescue PCS. Importantly, median postoperative survival following rescue PCS was only 1.99 years compared to 6.18 years following primary EPCS (p < 0.001).
Portal Systemic Encephalopathy
Table 5 shows data on PSE in the two groups of patients. Calculations of the incidence of PSE are based on patients who were discharged from the index hospitalization and survived more than 30 days after study entry since deaths on or before 30 days were considered indeterminate and unrelated to PSE. As we have reported previously, the incidence of PSE was 35% in the primary EST group and 15% in the primary EPCS group (p = 0.001).23 The difference in incidence of PSE was even greater when the primary EPCS group with its 15% PSE incidence was compared to the EST-rescue PCS group in which the PSE incidence was 43% (p < 0.001). Furthermore, as shown in Table 5, the number of episodes of PSE per patient and per year and the number of hospital readmissions per patient and per year were all significantly more frequent in the EST-rescue PCS group than in the EPCS group (p < 0.001). Additionally, the EST-rescue PCS patients with PSE had a median survival from the time of study entry of 3.44 years, which was longer than the 2.45 years of survival of the patients free of PSE, but the difference was not significant. In contrast, the patients in the primary EPCS group had a significantly longer survival than those in the EST-rescue PCS group (p < 0.001), and their median survival was 5.18 years for those with PSE and 10.43 years in those free of PSE (p < 0.001).
Dietary indiscretion with regard to protein restriction was the most frequent cause of recurrent PSE in both groups of patients. Portal hypertension-related UGI bleeding, usually from BEV, was the main cause of PSE in 23% of the episodes of PSE in the EST-rescue PCS group and was a contributing cause in an additional 16%. PSE episodes occurred more frequently prior to performance of rescue PCS than after rescue PCS. UGI bleeding was infrequently responsible for PSE in patients randomized to EPCS, occurring in only 8% of the patients even though they survived more than twice as long as the EST-rescue PCS patients (p < 0.001).
Change in Liver Function
Improvement or worsening of liver function was determined by serial quantitative measurements of Child’s risk class monthly during the first year after study entry and every 3 months thereafter. An increase or decrease in two or more Child’s class points reflected, respectively, improvement or worsening of liver function. Table 6 presents a summary of yearly changes in Child’s risk class using Child’s class on study entry as a baseline and combining Child’s classes A, B, and C. Results in patients randomized to EPCS are compared to results in the EST-rescue PCS patients. In every year, there was a statistically significant difference between the EPCS group and the EST-rescue PCS group, with the patients randomized to EPCS having more improvement and less worsening of liver function than the patients in the EST-rescue PCS group (p = 0.008 to <0.001). Overall, the 1- to 5-year average change in Child’s classes comparing EPCS versus EST-rescue PCS, respectively, showed improvement in 59% versus 32% and worsening in 10% versus 33% (p < 0.001). The differences in liver function between the EPCS and EST-rescue PCS groups were particularly striking in Child’s class C where improvement in liver function was most important. Five years after entry in the RCT, liver function had improved in 94% of the EPCS group compared to 65% in the EST-rescue PCS group, and liver function had worsened in 4% of the EPCS group compared to 30% of the EST-rescue PCS group. The difference in favor of EPCS was significant (p < 0.001).
Quality of Life Score
Table 7 summarizes data on QOL for 5 years in the 105 patients randomized to EPCS and the 50 patients who failed EST and underwent rescue PCS. QOL score was based on nine criteria shown at the bottom of Table 8. In the comparison, a lower score indicates a better QOL. Overall, during each year and for the entire 5-year period of study, QOL was significantly better, i.e., the QOL score was lower in the EPCS group than in the EST-rescue PCS group (p < 0.001).
Direct Costs of Care
Table 8 summarizes the total charges over a 10-year period for hospitalization and outpatient care in thousands of US dollars in patients randomized to EPCS and those randomized to EST-rescue PCS. The mean grand total charges over the entire length of the study were $150,400 in the EPCS patients and $263,600 in the EST-rescue PCS patients, a highly significant difference (p < 0.001). More importantly, the mean grand total charges per year amounted to $39,200 in the EPCS patients and $216,700 in the EST-rescue PCS patients, 5.5 times greater (p < 0.001).
Discussion
Comment is warranted regarding the use of EST rather than EVL in this RCT. In 1988 when the San Diego BEV Study was initiated, EST was a mainstay of therapy of BEV and the sole form of endoscopic therapy in use. When EVL was introduced generally, as well as at our institution, we were well into our RCT and our investigators and senior advisors made the unanimous decision not to change from EST to EVL. That decision has received strong support from studies published in 2003, 2005, and 2006 that have questioned replacement of EST by EVL. In a survey reported in 2003 of 93 gastroenterologists who treated 725 patients with BEV, EST was used more frequently than EVL for control of BEV and as frequently as EVL for initial control of acute bleeding.11 Trials published in 2005 and 1999 reported a significantly higher failure rate with band ligation of actively bleeding varices and an overall higher recurrence rate of varices treated by EVL.12,30 Moreover, EST has been reported to be more cost-effective if active variceal hemorrhage is present at the index endoscopy procedure, as was the case in our RCT.30 It is noteworthy that none of nine randomized clinical trials summarized in 2005 observed a statistically significant difference in survival rate between EVL and EST.12 In a meta-analysis of emergency EST in 40 trials involving 4031 patients reported by Triantos et al.10 in 2006, there was no statistically significant difference in survival rate between EVL and EST. The authors concluded that “the conclusive evidence for substituting banding ligation or the combination of vasoconstrictors with sclerotherapy as better therapeutic approaches has not been provided in randomized trials. Sclerotherapy can remain a gold standard in variceal bleeding….”
It is widely agreed that portal-systemic shunts are very effective in controlling BEV. The results of our RCT confirm such effectiveness since both EPCS and rescue PCS promptly and permanently controlled BEV in every patient. Nevertheless, according to numerous statements in the literature, surgical shunts control bleeding at the expense of an unacceptably high rate of PSE as well as progressive liver failure, and that is the main reason why portal-systemic shunts have been relegated to a secondary salvage role for use solely as a last resort when endoscopic and pharmacologic measures have failed.4,13–21 The results of our RCT, which involved unselected, consecutive cirrhotic patients with all degrees of liver dysfunction, including patients in Child’s class C, contradict the widely held beliefs about the appropriate role of portal-systemic shunts. According to our findings which have been reported in detail recently,23 the incidence of PSE following EPCS was significantly lower (15%) than the incidence following primary EST (35%) or after EST with rescue PCS (43%). The protocol of our RCT describes the requirements for achieving a low incidence of PSE.23 These are: (1) diagnosis and EPCS within 24 h of onset of BEV; (2) operation by surgeons experienced in portal hypertension surgery; (3) postoperative care in an ICU by trained and experienced nurses and physicians; and (4) regular, long-term follow-up that includes concerted efforts to promote abstinence from alcohol and repeated emphasis on reasonable restriction of dietary protein intake. It is our conviction that these requirements can be fulfilled by most trained surgeons and by most general hospitals in the USA.
Regarding the matter of post-shunt liver failure, the concept that direct portacaval shunts cause liver failure because of diversion of essential portal blood flow began over a century ago with the animal experiments of Eck and Hahn and associates in Pavlov’s laboratory31,32 and has been suggested repeatedly but not substantiated since then.33–35 The concept has led to the invention of a number of operations that are purported to maintain portal blood flow to the liver while overcoming portal hypertension. These include distal splenorenal shunt, small-diameter prosthetic, H-graft portacaval shunt, and small-diameter direct side-to-side portacaval shunt. However, the concept is contradicted by two important hemodynamic facts. The first is that whether or not a PCS is constructed, BEV arise as a consequence of progressive diversion of a substantial portion of venous blood flow away from the liver and into portal-systemic collaterals so that, with regard to creation of a PCS, the cirrhotic liver with BEV is markedly different from the normal liver. The second important hemodynamic fact is that a fundamental physiologic response to diversion of portal venous flow is a compensatory increase in hepatic arterial blood flow to the liver.36–38 It is not possible by any currently available practical method to predict the adequacy of hepatic arterial compensation prior to performance of a PCS. Substantial data from preoperative and intraoperative measurements of both pressure and blood flow in the portal vein in large numbers of patients have failed to show a correlation between any hemodynamic measurements performed prior to PCS and survival, hepatic function, or development of PSE after PCS.36–40 Our studies of portal vein hemodynamics before PCS showed no statistically significant correlation between pre-shunt maximum perfusion pressure and post-shunt survival, liver function, hepatic failure, or development of PSE.38 It is noteworthy that Burchell and colleagues in their extensive intraoperative hemodynamic studies observed the largest post-shunt increments in compensatory hepatic arterial flow following side-to-side PCS, the procedure performed in 99 of the 105 EPCS patients in our RCT. In the final analysis, the long-term improvement in liver function following EPCS observed in the current trial provides the most meaningful and objective information regarding the effect of portal venous flow diversion on the cirrhotic liver. Each year for 5 years after EPCS, liver function improved in 59–65% of patients, and liver function declined in only 8–11%.
The San Diego BEV Study provided a unique opportunity in a RCT to compare EPCS, a treatment that is infrequently used today, with a conventional treatment regimen consisting of rescue PCS following failure of EST to control BEV. Not only did EPCS prove to be superior to EST, but also, by every measure of effectiveness, EPCS proved to be significantly better than the combination of EST with rescue PCS. How can this striking difference be explained? A likely explanation is that patients who require rescue PCS are much more severely ill than patients who undergo a diagnostic workup and a definitive operation within 24 h of the onset of bleeding. They are poorer candidates for operation or, for that matter, for any other form of rescue therapy. There is little doubt that persistent variceal bleeding, repeated readmissions to the hospital, and repeated bouts of PSE in the EST patients take their toll. In point of fact, by the time rescue PCS was required, many of the patients had experienced a decline in liver function reflected by a negative change in Child’s risk class. Furthermore, one third of the patients who failed EST died before having the opportunity to undergo rescue PCS, a common occurrence in programs that treat cirrhotic patients with BEV.
Kahn et al.,5 in their extensive review of emergency treatment of BEV, identified serious shortcomings in many of the reported studies. The San Diego BEV Study was designed to overcome these shortcomings and was unique in the following respects: (1) the 211 patients with acute BEV were unselected and consecutive; (2) physicians from four California counties with a population of 8.5 million agreed to participate in the study; (3) the diagnostic workup was completed rapidly in a mean 3.1–4.4 h, entirely at the bedside in the ICU; (4) unlike any reported study to date, definitive treatment with EST or EPCS was started within 8 h of study entry in 208 of 211 patients; (5) the surgeons and gastroenterologists were experienced senior faculty physicians; (6) follow-up was 100%, was regular, and extended for 9.4 to more than 10 years or until death; (7) concerted, organized, and often successful efforts were made to control dietary protein intake and alcoholism; (8) PSE was determined and prevented according to a clearly defined protocol by a “blinded” senior gastroenterologist; and (9) consistent with our past experience following EPCS, only 2 of 105 patients developed shunt occlusion, which prevented recurrent BEV and PSE.
Conclusion
In this RCT of emergency treatment of acute BEV in 211 unselected, consecutive patients with cirrhosis of all grades of severity, EPCS was strikingly superior to EST as well as to the combination of EST and rescue PCS in regard to all outcome measures, specifically control of bleeding, survival, incidence of PSE, improvement in liver function, quality of life, and cost of care. These results contradict the widespread belief that PCS, otherwise known as total shunt, is associated with a high incidence of PSE and causes liver failure. Moreover, these results call into question the widespread practice of relegating PCS solely to salvage failure of endoscopic therapy of BEV and strongly support the use of EPCS as the first line of emergency treatment of BEV in cirrhosis.
Abbreviations
- BEV:
-
Bleeding esophageal varices
- EST:
-
Endoscopic sclerotherapy
- EPCS:
-
Emergency portacaval shunt
- PCS:
-
Portacaval shunt
- UGI:
-
Upper gastrointestinal
- ICU:
-
Intensive care unit
- PRBC:
-
Packed red blood cells
- PSE:
-
Portal-systemic encephalopathy
- EVL:
-
Endoscopic variceal ligation
- QOL:
-
Quality of life
References
Graham DY, Smith H. The course of patients after variceal hemorrhage. Gastroenterology 1981; 80:800–809.
Smith JL, Graham DY. Variceal hemorrhage: a critical evaluation of survival analysis. Gastroenterology 1982; 82:968–973.
Burroughs AK, Mezzanotte G, Phillips A, McCormick PA, McIntyre N. Cirrhotics with variceal hemorrhage: the importance of the time interval between admission and the start of analysis for survival and rebleeding rates. Hepatology 1969; 9:801–807.
Bornman PC, Krige JE, Terblanche J. Management of esophageal varices. Lancet 1994; 353:1079–1084.
Khan S, Tudur Smith C, Williamson P, Sutton R. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No. CD000553. doi:10.1002/14651858. CD000553. Pub 2.
Orloff MJ, Duguay LR, and Kosta LD. Criteria for selection of patients for emergency portacaval shunt. Am J Surg 1977; 134:146–152.
Mikkelsen WP. Therapeutic portacaval shunt. Preliminary data on controlled trial and morbid effects of acute hyaline necrosis. Arch Surg 1974; 108:302–305.
Terblanche J, Burroughs AK, Hobbs KEF. Controversies in the management of bleeding oesophageal varices. N Engl J Med 1989; 320:1393–1398.
D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995; 22:332–354.
Triantos CK, Goulis J, Patch D, Papatheodoridis GV, Leandro G, Samonakis D, Cholongitas E, and Burroughs AK. An evaluation of emergency sclerotherapy of varices in randomized trials: looking the needle in the eye. Endoscopy 2006; 38:797–808.
Sorbi D, Gostout CJ, Peura D, Johnson D, Lanza F, Foutch PG, Schleck CD, Zinsmeister AR. An assessment of the management of acute bleeding varices: a multicenter prospective member-based study. Am J Gastroenterol 2003; 98:2424–2434.
Krige JE, Shaw JM, Bornman PC. The evolving role of endoscopic treatment of esophageal varices. Wld J Surg 2005; 29:966–973.
Garcia N Jr, Sanyal AJ. Portal hypertension. Clin Liver Dis. 2001; 5:1–26.
Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. Am J Gastroenterol. 1997; 92:1081–1091.
Abraldes JG, Angermayr B, Bosch J. The management of portal hypertension. Clin Liver Dis. 2005; 9:685–713.
Khan S, Tudur Smith C, Williamson P, Sutton R. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database Syst Rev. 2006:CD000553.
Grace ND. The side-to-side portacaval shunt revisited. N Engl J Med. 1994; 330:208–209.
Pagliaro L, Burroughs AK, Sorensen TI, Lebrec D, Morabito A, D’Amico G, Tine F. Therapeutic controversies and randomized controlled trials (RCTs): prevention of bleeding and rebleeding in cirrhosis. Gastroenterology International 1989; 2:71–84.
Wright AS, Rikkers LF. Current management of portal hypertension. J Gastrointest Surg. 2005; 9:992–1005.
Rikkers LF, Jin G. Emergency shunt. Role in the present management of variceal bleeding. Arch Surg. 1995; 130:472–477.
Capussotti L, Vergara V, Polastri R, Bouzari H, Galatola G. Liver function and encephalopathy after partial vs direct side-to-side portacaval shunt: A prospective randomized clinical trial. Surgery 2000; 127:614–621.
Orloff MJ, Isenberg JI, Wheeler HO, Haynes KS, Jinich-Brook H, Rapier R, Vaida F, Hye RJ. Randomized trial of emergency endoscopic sclerotherapy versus emergency portacaval shunt for acutely bleeding esophageal varices in cirrhosis. J Am Coll Surg 2009; 209:25–45.
Orloff MJ, Isenberg JI, Wheeler HO, Haynes KS, Jinich-Brook H, Rapier R, Vaida F, Hye RJ. Portal-systemic encephalopathy in a randomized controlled trial of endoscopic sclerotherapy versus emergency portacaval shunt treatment of acutely bleeding esophageal varices in cirrhosis. Ann Surg 2009; 250:598–610.
Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001; 285:1987–1991.
Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gotzsche PC, Lang T. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001; 134:663–694.
Orloff MJ, Orloff MS, Orloff SL, Rambotti M, Girard B. Three decades of experience with emergency portacaval shunt for acutely bleeding esophageal varices in 400 unselected patients with cirrhosis of the liver. J Am Coll Surg 1995; 180:257–272.
Child III, CG, Turcotte JG. Surgery and portal hypertension. In: The Liver and Portal Hypertension. Edited by CG Child III. W.B. Saunders, Philadelphia, pp. 1–85, 1964.
Campbell DP, Parker DE, Anagnostopoulos CE. Survival prediction in portacaval shunt: a computerized statistical analysis. Am J Surg 1973; 126:748–751.
Orloff MJ, Orloff SL, Orloff MS. Portacaval shunts: side-to-side and end-to-side. In: Atlas of Upper Gastrointestinal and Hepato-Pancreato-Biliary Surgery. P-A Clavien, MG Saar, Y Fong, eds. Springer-Verlag, Berlin Heidelberg, 2007, pp. 687–702.
Gralnek IM, Jensen DM, Kovacs TOG, Jutabha R, Gornbein J, Cheng S, King J, Jensen ME. The economic impact of esophageal variceal hemorrhage: cost-effectiveness implications of endoscopic therapy. Hepatology 1999; 29:44–50.
Eck NV. Kvoprosu o pbrevyazkie vorotnois veni. Prdvaritblmoye soobshtshjdmye. Voen Med Zh 1877; 130:1–2.
Hahn M, Massen O, Nencki M, Pavlov J. Die Eck’sche Fistel zwischen der untern Hohlvene und der Pfortader und ihre Folgen fuer den Organismus. Arch Exp Pathol Pharmacol 1893; 32:161–210.
Reynolds TB, Mikkelson WP, Redeker AG, Yamahiro HS. The effect of a side-to-side portacaval shunt on hepatic hemodynamics in cirrhosis. J Clin Invest 1962; 41:1242–1248.
Ferguson JD. Hemodynamics in surgery for portal hypertension. Ann Surg 1963; 158:383–386.
Price JB, Voorhees AB Jr, Britton RC. Operative hemodynamic studies in portal hypertension. Arch Surg 1967; 95:843–852.
Burchell AR, Moreno AH, Panke WF, Nealon TF. Hemodynamic variables and prognosis following portacaval shunt. Surg Gynecol Obstet 1974; 138:359–369.
Burchell AR, Moreno AH, Panke WF, Nealon TF Jr. Hepatic artery flow improvement after portacaval shunt: A single hemodynamic clinical correlate. Ann Surg 1976; 184:289–300.
Charters AC, Brown BN, Sviokla S, Knox D, Orloff MJ. The influence of portal perfusion on the response to portacaval shunt. Am J Surg 1975; 130:226–232.
Price JB Jr, Britton RC, Voorhees AB Jr. The significance and limitations of operative hemodynamics in portal hypertension. Arch Surg 1967; 95:843–852.
Steegmuller KW, Marklin H-M, Hollis HW Jr. Intraoperative hemodynamic investigations during portacaval shunt. Arch Surg 1984; 119:269–273.
Acknowledgment
This study was supported by grant 1R01 DK41920 from the National Institutes of Health and a grant from the Surgical Education and Research Foundation (Clinicaltrials.gov NCT 00690027). We thank the many residents in the Department of Medicine and the Department of Surgery at UCSD Medical Center who played a major role in the care of patients in this study. We thank Professors Harold O Conn, Haile T Debas, and Peter Gregory who served voluntarily as an External Advisory and Monitoring Committee. We thank the many physicians practicing in the counties of San Diego, Imperial, Orange, and Riverside who helped with patient recruitment, referral, and long-term follow-up.
Conflict of Interest
There was no conflict of interest relevant to this article on the part of any of the authors and no financial interests, relationships, or affiliations.
Author Contributions
Orloff, Isenberg, Wheeler contributed to the study conception and design. Acquisition of data was done by Orloff, Isenberg, Haynes, Jinich-Brook, Rapier, Hye. Analysis and interpretation of data was done by Orloff, Isenberg, Vaida. Orloff, Haynes, Vaida, Hye took care of the drafting of the manuscript. Orloff, Haynes, Jinich-Brook, Vaida, Hye did the critical revision.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 51st Annual Meeting of the Society for Surgery of the Alimentary Tract during Digestive Disease Week, New Orleans, Louisiana, May 1–5, 2010.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Orloff, M.J., Isenberg, J.I., Wheeler, H.O. et al. Emergency Portacaval Shunt Versus Rescue Portacaval Shunt in a Randomized Controlled Trial of Emergency Treatment of Acutely Bleeding Esophageal Varices in Cirrhosis—Part 3. J Gastrointest Surg 14, 1782–1795 (2010). https://doi.org/10.1007/s11605-010-1279-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-010-1279-7