Abstract
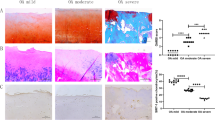
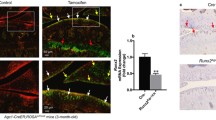
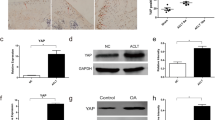
This study investigated the effects of SIRT1 gene knock-out on osteoarthritis in mice, and the possible roles of SREBP2 protein and the PI3K/AKT signaling pathway in the effects. Mice were randomly divided into a normal group and a SIRT1 gene knock-out group (6 mice in each group). In these groups, one side of the knee anterior cruciate ligament was traversed, and the ipsilateral medial meniscus was cut to establish an osteoarthritis model of knee joint. The countralateral synovial bursa was cut out, serving as controls. The knee joint specimens were then divided into four groups: SIRT1+/+ control group (group A, n=6); SIRT1+/+ osteoarthritis group (group B, n=6); SIRT1–/– control group (group C, n=6); SIRT1–/– osteoarthritis group (group D, n=6). HE staining, Masson staining, Safranin O-Fast Green staining and Van Gieson staining were used to observe the morphological changes in the articular cartilage of the knee. Immunohistochemical staining was employed to detect the expression of SIRT1, SREBP2, VEGF, AKT, HMGCR and type II collagen proteins. SA-β-gal staining was utilized to evaluate chondrocyte aging. The results showed clear knee joint cartilage destruction and degeneration in the SIRT1–/– osteoarthritis group. The tidal line was twisted and displaced anteriorly. Type II collagen was destroyed and distributed unevenly. Compared with the SIRT1+/+ osteoarthritis group and SIRT1–/– control group, SIRT1 protein expression was not obviously changed in the SIRT1–/– osteoarthritis group (P>0.05), while the expression levels of the SREBP2, VEGF and HMGCR proteins were significantly increased (P<0.05) and the levels of AKT and type II collagen proteins were significantly decreased (P<0.05). SIRT1 gene knock-out may aggravate cartilage degeneration in osteoarthritis by activating the SREBP2 protein-mediated PI3K/AKT signalling pathway, suggesting that SIRT1 gene may play a protective role against osteoarthritis.
Similar content being viewed by others
References
Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part I: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage, 2010,18(4):476–499
Ghosh S, Zhou Z. SIRTain regulators of premature senescence and accelerated aging. Protein Cell, 2015,6(5):322–333
Goldstein JL, Rawson RB, Brown MS, et al. Mutant mammalian cells as tools to delineate the sterol regulatory element binding protein pathway for feedback regulation of lipid synthesis. Arch Biochem Biophys, 2002,397(2):39–48
Li LC, Varghese Z, Moorhead JF, et al. Cross-talk between TLR4-MyD88-NF-NB and SCAP-SREBP2 pathways mediates macrophage foam cell formation. Am J Physiol Heart Circ Physiol, 2013,304(6): H874–H884
Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature, 2004,429(6993):771–776
Nishi J, Minamino T, Miyauchi H, et al. Vascular endothelial growth factor receptor-1 regulates postnatal angiogenesis through inhibition of the excessive activation of Akt. Circ Res, 2008,103(3):261–268
Sun P, Yu H, Zhang WQ, et al. Mechanism of the apoptosis of human gastric cancer cell line SGC7901 induced by vascular endothelial growth factor siRNA. J Med Postgra (Chinese), 2011,4(24):350–353
Yeung F, Hoberg JE, Ramsey, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J, 2004,23(12):2369–2380
Ren T, Huang C, Cheng M. Dietary blueberry and bifidobacteria attenuate nonalcoholic fatty liver disease in rats by affecting SIRT1-mediated signaling pathway. Oxid Med Cell Longev, 2014,2014:469059
He W, Dai C. Key fibrogenic signaling. Curr Pathobiol Rep, 2015,3(2):183–192
Yu F, Lei M, Zeng H, et al. Construction of elderly SIRT 1 gene knockout mouse models of knee osteoarthritis. Chin J Tissue Eng Res (Chinese), 2015,19(49):7895–7901
Fujita N, Matsushita T, Ishida K, et al. Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions. J Orthop Res, J Orthop Res. 2011,29(4):511–515
Takayama K, Ishida K, Matsushita T, et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum, 2009,60(9):2731–2740
Lotz M, Hashimoto S, Kuhn K. Mechanisms of chondrocyte apoptosis.Osteoarthritis Cartilage, 1999,7(1):389–391
Courties A, Gualillo O, Berenbaum F, et al. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015,23(11):1955–1965
Kontny E, Janicka I, Skalska U, et al. The effect of multimeric adiponectin isoforms and leptin on the function of rheumatoid fibroblast-like synoviocytes. Scand J Rheumatol, 2015,44(5):363–368
Manolagas SC, Almeida M. Gone with the Wnts:ß-catenin, Tcell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol, 2007,21(11):2605–2614
Simic P, Zainabadi K, Bell E, et al. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating ß-catenin. EMBO Mol Med, 2013,5(3):430–440
Walsh DA, Bonnet CS, Turner EL, et al. Angiogenesis in the synovium and at the osteochondral junction in oteoarthritis. Osteoarthritis Cartilage, 2007,15(7):743–751
Luo X, Li HX, Yang GS. Sequential expreßsion of wnt/ß-catenin signal pathway related genes and adipocyte transcription factors during porcine adipose tissue development. Chin J Biotechnol (Chinese), 2008,24(5):746–753
Yao L, Xiao Y, Liu SP, et al. TLR4/NF-kB signal pathway mediates the proinflammatory effect of the obesity serum. Chin J Arterioscler (Chinese), 2010,18(11):873–877
Xiang XX, Chen L, Wang JH, et al. Role of Wnt/ß-catenin signaling in aging of mesenchymal stem cells of rats. Zhejiang Da Xue Xue Bao Yi Xue Ban (Chinese), 2011,40(6):630–640
Kostopoulou F, Gkretsi V, Malizos KN, et al. Central role of SREBP-2 in the pathogenesis of osteoarthritis. PLoS One, 2012,7(5):e 35753
Lewis CA, Griffiths B, Santos CR, et al. Regulation of the SREBP transcription factors by mTORC1. Biochem Soc Trans, 2011,39(2):495–499
Sperka T, Wang J, Rudoph KL. DNA damage checkpoints in stem cells, aging and cancer. Nat Rev Mol Cell Biol, 2012,13(9):579–590
Lim JH, Lee YM, Chun YS, et al. Sirtuin1 modulates cellular reponses to hypoxia by deacetylating hypoxia-indueible factor lalpha. Mol Cell, 2010,38(6):864–78
Wang M, Shen J, Jin H, et al. Recent process in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci, 2011,1240:61–9
Zhang C, Huang L, Luo QF, et al. Ezetimibe inhibit hyperacetylation of SREBP-1c in lipid-loaded cells derived from VSMC required for SIRT1. Chin J Arterioscler (Chinese), 2012,20(6):519–522
Kamei Y, Miura S, Suganami T, et al. Regulation of SREBP1c gene expression in skeletalmuscle: role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factor. Endocrinology, 2008,149:2293–2305
Gao YX, Zhang Y, Liu Y, et al. SREBP-1c is involved in preventive effect of resveratrol on pathogenesis of nonalcoholic fatty liver disease in rats. Disan Junyi Daxue Xuebao (Chinese), 2015,37(17):1704–1708
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from the National Natural Science Foundation of China (No. 81272032) and the Research and Development Projects of Shenzhen of China (Nos. JCYJ20150403091443275).
Rights and permissions
About this article
Cite this article
Yu, F., Zeng, H., Lei, M. et al. Effects of SIRT1 gene knock-out via activation of SREBP2 protein-mediated PI3K/AKT signaling on osteoarthritis in mice. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 36, 683–690 (2016). https://doi.org/10.1007/s11596-016-1645-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-016-1645-0