Abstract
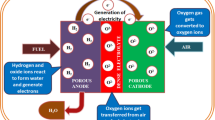
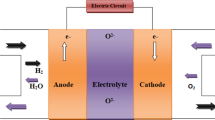
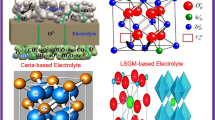
A solid oxide fuel cell (SOFC) is an energy conversion device, which directly converts chemical fuels (e.g., H2, CO, and CxHy) into electricity and heat with high efficiency up to about 90 %. The main by-product CO2 when hydrocarbons used as fuels can be sequestrated or subsequently chemically transformed into chemical fuels (e.g., CO) by electrolysis using renewable energy sources, such as solar and wind. The state-of-the-art nickel and yttria-stabilized zirconia (YSZ) composite (Ni-YSZ) anode is deactivated in the presence of ppm level of H2S and forming coke in hydrocarbons at the operating temperature of SOFCs. Currently, mixed ion and electron conducting (MIEC) metal oxides are being considered as alternatives for Ni-YSZ anode in SOFCs. In this review, we report the recent development of MIEC metal oxides anodes for advanced SOFCs.





















Similar content being viewed by others
References
Keeling CD (1957) Variations in concentration and isotopic abundances of atmospheric carbon dioxide. Proceedings of the Conference on Recent Research in Climatology, H Craig, Ed (Committee on Research in Water Resources and University of California, Scripps Institution of Oceanography, La Jolla, CA, 1957):43–49
Keeling CD, Whorf TP, Wahlen M, Jvd P (1995) Interannual extremes in the rate of rise of atmospheric carbon dioxide since 1980. Nature 375:666–670. doi:10.1038/375666a0
Hansen J, Johnson D, Lacis A, Lebedeff S, Lee P, Rind D, Russell G (1981) Climate impact of increasing atmospheric carbon dioxide. Science 213:957–966. doi:10.1126/science.213.4511.957
Hansen J, Ruedy R, Glascoe J, Sato M (1999) GISS analysis of surface temperature change. J Geophys Res 104:30997–31022. doi:10.1029/1999JD900835
Hansen J, Ruedy R, Sato M, Lo K (2010) Global surface temperature change. Rev Geophys 48:RG4004. doi:10.1029/2010RG000345
Hansen JE, Lebedeff S (1987) Global trends of measured surface air temperature. J Geophys Res 92:13345–13372. doi:10.1029/JD092iD11p13345
Hansen JE, Ruedy R, Sato M, Imhoff M, Lawrence W, Easterling D, Peterson T, Karl T (2001) A closer look at United States and global surface temperature change. J Geophys Res 106:23947–23963. doi:10.1029/2001JD000354
Chen X, Tung K-K (2014) Varying planetary heat sink led to global-warming slowdown and acceleration. Science 345(6199):897–903. doi:10.1126/science.1254937
Bristish Petroleum (2013) British petroleum statistical review of world energy. http://www.bp.com/content/dam/bp/pdf/statistical-review/statistical_review_of_world_energy_2013.pdf
Conway BE, Birss V, Wojtowicz J (1997) The role and utilization of pseudocapacitance for energy storage by supercapacitors. J Power Sources 66:1–14. doi:10.1016/S0378-7753(96)02474-3
Badi S-P, Wagemaker M, Ellis BL, Singh DP, Borghols WJH, Kan WH, Ryan DH, Mulder FM, Nazar LF (2011) Direct synthesis of nanocrystalline Li0.90FePO4: observation of phase segregation of anti-site defects on delithiation. J Mater Chem 21:10085–10093. doi:10.1039/C0JM04378H
Ellis B, Kan WH, Makahnouk WRM, Nazar LF (2007) Synthesis of nanocrystals and morphology control of hydrothermally prepared LiFePO4. J Mater Chem 17:3248–3254. doi:10.1039/b705443m
Kan WH (2009) Study of the morphology control and solid solution behaviour of Olivine LiMPO4 (M = Fe, Mn, and Co). University of Waterloo
Lee KT, Kan WH, Nazar LF (2009) Proof of intercrystallite ionic transport in LiMPO4 electrodes (M = Fe, Mn). J Am Chem Soc 131:6044–6045. doi:10.1021/ja8090559
Singhal SC, Kendall K (2003) High temperature solid oxide fuel cells fundamentals, design and applications. Elsevier, Oxford
Larminie J, Dicks A (2003) Fuel cell systems explained, 2nd edn. Wiley, New York
O’Hayre R, Cha S-W, Colella W, Prinz FB (2009) Fuel cell fundamentals. Wiley, New York
Ishihara T (2009) Perovskite oxide for solid oxide fuel cells. Springer, Berlin
Wu Z-S, Parvez K, Feng X, Müllen K (2013) Graphene-based in-plane micro-supercapacitors with high power and energy densities. Nat Commun 4:2487/2481–2487/2488. doi:10.1038/ncomms3487
Narayan SR, Valdez TI (2008) High-energy portablef fuel cell power sources. Electrochem Soc Interface 17:40–45
Barnett SA (2003) Handbook of fuel cell technology, vol 4. Wiley, New York
Goodenough JB, Huang Y-H (2007) Alternative anode materials for solid oxide fuel cells. J Power Sources 173:1–10. doi:10.1016/j.jpowsour.2007.08.011
Singhal SC (2000) Advances in solid oxide fuel cell technology. Solid State Ionics 135:305–313. doi:10.1016/S0167-2738(00)00452-5
Jacobson AJ (2010) Materials for solid oxide fuel cells. Chem Mater 22:660–674. doi:10.1021/cm902640j
Atkinson A, Barnett S, Gorte RJ, Irvine JTS, McEvoy AJ, Mogensen M, Singhal SC, Vohs J (2004) Advanced anodes for high-temperature fuel cells. Nat Mater 3:17–27. doi:10.1038/nmat1040
Winkler W, Lorenz H (2002) The design of stationary and mobile solid oxide fuel cell-gas turbine systems. J Power Sources 105:222–227. doi:10.1016/S0378-7753(01)00943-0
Wray P (2010) Bloom bursts into SOFC business. Am Ceram Soc Bull 89:25–25
Endo M (2012) The future perspective of SOFC. Nenryo Denchi 12:60–66
Steele BCH (2000) Materials for IT-SOFC stacks 35 years R&D: the inevitability of gradualness? Solid State Ionics 134:3–20. doi:10.1016/S0167-2738(00)00709-8
Williams MC, Strakey J, Sudoval W (2006) U.S. DOE fossil energy fuel cells program. J Power Sources 159:1241–1247. doi:10.1016/j.jpowsour.2005.12.085
Park S, Vohs JM, Gorte RJ (1999) Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 404:265–267. doi:10.1038/35005040
Murray EP, Tsai T, Barnett SA (1999) A direct-methane fuel cell with a ceria-based anode. Nature 400:649–651. doi:10.1038/23220
Huang Y-H, Dass RI, Xing Z-L, Goodenough JB (2006) Double perovskites as anode materials for solid-oxide fuel cells. Science 312:254–257. doi:10.1126/science.1125877
Skinner SJ, Kilner JA (2000) Oxygen diffusion and surface exchange in La2-xSrxNiO4+δ. Solid State Ionics 135:709–712. doi:10.1016/S0167-2738(00)00388-X
Eguchi K, Setoguchi T, Inoue T, Arai H (1992) Electrical properties of ceria-based oxides and their application to solid oxide fuel cells. Solid State Ionics 52:165–172. doi:10.1016/0167-2738(92)90102-U
Huang K, Tichy RS, Goodenough JB (1998) Superior perovskite oxide-ion conductor; strontium- and magnesium-doped LaGaO3: I, phase relationships and electrical properties. J Am Ceram Soc 81(10):2565–2575. doi:10.1111/j.1151-2916.1998.tb02662.x
Mogensen M, Sammes NM, Tompsett GA (2000) Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ionics 129:63–94. doi:10.1016/S0167-2738(99)00318-5
Fabbri E, Pergolesi D, Traversa E (2010) Materials challenges toward proton-conducting oxide fuel cells: a critical review. Chem Soc Rev 39(11):4355–4369. doi:10.1039/b902343g
Thangadurai V, Kan WH, Mirfakhraei B, Bhella SS, Trinh TT (2011) Materials for proton conducting solid oxide fuel cells (H-SOFCs). ECS Trans 35:483–492. doi:10.1149/1.3570024
Suzuki T, Hasan Z, Funahashi Y, Yamaguchi T, Fujishiro Y, Awano M (2009) Impact of anode microstructure on solid oxide fuel cells. Science 325:852–855. doi:10.1126/science.1176404
Wilson JR, Kobsiriphat W, Mendoza R, Chen H-Y, Hiller JM, Miller DJ, Thornton K, Voorhees PW, Adler SB, Barnett SA (2006) Three-dimensional reconstruction of a solid-oxide fuel-cell anode. Nat Mater 5:541–544. doi:10.1038/nmat1668
Simwonis D, Tietz F, Stover D (2000) Nickel coarsening in annealed Ni/8YSZ anode substrates for solid oxide fuel cells. Solid State Ionics 132:241–251. doi:10.1016/S0167-2738(00)00650-0
Yang L, Cheng Z, Liu M, Wilson L (2010) New insights into sulfur poisoning behavior of Ni-YSZ anode from long-term operation of anode-supported SOFCs. Energy Environ Sci 3:1804–1809. doi:10.1039/C0EE00386G
Yang L, Choi Y, Qin W, Chen H, Blinn K, Liu M, Liu P, Bai J, Tyson TA, Liu M (2011) Promotion of water-mediated carbon removal by nanostructured barium oxide/nickel interfaces in solid oxide fuel cells. Nat Commun 2:1359/1351–1359/1359. doi:10.1038/ncomms1359
Shao Z, Haile SM (2004) A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431:170–173. doi:10.1038/nature02863
Liu J, Co AC, Paulson S, Birss VI (2006) Oxygen reduction at sol–gel derived La0.8Sr0.2Co0.8Fe0.2O3 cathodes. Solid State Ionics 117:377–387. doi:10.1016/j.ssi.2005.11.005
Wang S, He Q, Liu M (2009) Promising Ni-Fe-LSGMC anode compatible with lanthanum gallate electrolyte. Electrochim Acta 54:3872–3876. doi:10.1016/j.electacta.2009.02.002
Smith TR, Wood A, Birss VI (2009) Effect of hydrogen sulfide on the direct internal reforming of methane in solid oxide fuel cells. Appl Catal A 354:1–7. doi:10.1016/j.apcata.2008.10.055
Mogensen M, Bentzen JJ (1989) Oxidation of methane on oxide electrodes at 800-1000 °C. Proc Electrochem Soc 89–11:99–110
Marina OA, Bagger C, Primdahl S, Mogensen M (1999) A solid oxide fuel cell with a gadolinia-doped ceria anode: preparation and performance. Solid State Ionics 123:199. doi:10.1016/S0167-2738(99)00111-3
Takahashi T, Iwahara H, Suzuki Y (1969) Proceedings of the third international symposium on fuel cells. bruxelles : presses academiques europeennes. Journees Int Etude Piles Combust 3:113–119
Steele BCH, Kelly I, Middleton H, Rudkin R (1988) Oxidation of methane in solid state electrochemical reactors. Solid State Ionics 28–30:1547–1552. doi:10.1016/0167-2738(88)90417-1
Zhan ZI, Barnett SA (2005) An octane-fueled solid oxide fuel cell. Science 308:844–847. doi:10.1126/science.1109213
Zhang C-J, Grass ME, McDaniel AH, DS C, Gabaly FE, Liu Z, McCarty KF, Farrow RL, Linne MA, Hussain Z, Jackson GS, Bluhm H, Eichhorn BW (2010) Measuring fundamental properties in operating solid oxide electrochemical cells by using in situ x-ray photoelectron spectroscopy. Nat Mater 9:944–949. doi:10.1038/nmat2851
Chueh WC, Hao Y, Jung WC, Haile SM (2012) High electrochemical activity of the oxide phase in model ceria–Pt and ceria–Ni composite anodes. Nat Mater 11:155–161. doi:10.1038/nmat3184
Handal HT, Thangadurai V (2013) Evaluation of chemical stability, thermal expansion coefficient, and electrical properties of solid state and wet-chemical synthesized Y and Mn-codoped CeO2 for solid oxide fuel cells. J Power Sources 243:458–471. doi:10.1016/j.jpowsour.2013.05.173
Li Q, Thangadurai V (2010) A comparative 2 and 4-probe DC and 2-probe AC electrical conductivity of novel co-doped Ce0.9−xRExMo0.1O2.1–0.5x (RE = Y, Sm, Gd; x = 0.2, 0.3). J Mater Chem 20:7970–7983. doi:10.1039/C0JM01324B
Mirfakhraei B, Birss V, Thangadurai V, Paulson S, Bere KE, Gitzhofer F (2011) Electrochemical performance and H2S poisoning study of Mo-doped ceria (CMO) SOFC anodes. ECS Trans 35:1727–1734. doi:10.1149/1.3570160
Chen Y, Bunch J, Jin C, Yang C, Chen F (2012) Performance enhancement of Ni-YSZ electrode by impregnation of Mo0.1Ce0.9O2+δ. J Power Sources 204:40–45. doi:10.1016/j.jpowsour.2012.01.019
Smart LE, Moore EA (2005) Solid state chemistry an introduction, 3rd edn. CRC Taylor & Francis, Prentice Hall
West AR (2009) Solid state chemistry and its applications. Wiley, New York
Tao S, Irvine JTS (2003) A redox-stable, efficient anode for solid-oxide fuel cells. Nat Mater 2:320–323. doi:10.1038/nmat871
Ruiz-Morales JC, Canales-Vazquez J, Savaniu C, Marrero-Lopez D, Zhou W, Irvine JTS (2006) Disruption of extended defects in solid oxide fuel cell anodes for methane oxidation. Nature 439(7076):568–571. doi:10.1038/nature04438
Yang L, Wang S, Blinn K, Liu M, Cheng Z, Liu M (2009) Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2–xYbxO3–δ. Science 326:126–129. doi:10.1126/science.1174811
Shima D, Haile SM (1997) The influence of cation non-stoichiometry on the properties of undoped and gadolinia-doped barium cerate. Solid State Ionics 97:443–455. doi:10.1016/S0167-2738(97)00029-5
Mirfakhraei B, Ramezanipour F, Paulson S, Birss V, Thangadurai V (2014) Effect of sintering temperature on microstructure, chemical stability, and electrical properties of transition metal or Yb-doped BaZr0.1Ce0.7Y0.1 M0.1O3−δ (M = Fe, Ni, Co, and Yb). Front Energy Res 2:9/1–9/10. doi:10.3389/fenrg.2014.00009
Du Y, Nowick AS (1996) Galvanic cell measurements on a fast proton conducting complex perovskite electrolyte. Solid State Ionics 91:85–91. doi:10.1016/S0167-2738(96)00409-2
Liang KC, Du Y, Nowick AS (1994) Fast high-temperature proton transport in nonstoichiometric mixed perovskites. Solid State Ionics 69:117–120. doi:10.1016/0167-2738(94)90399-9
Liang KC, Nowick AS (1998) Protonic conduction in nonstoichiometric Sr2(Bʹ1.1Nb0.9)O6 compounds. Electrochem Soc Proc 97–24:28–35
Liang KC, Nowick AS (1993) High-temperature protonic conduction in mixed perovskite ceramics. Solid State Ionics 61:77–81. doi:10.1016/0167-2738(93)90337-3
Nowick AS, Du Y, Liang KC (1999) Some factors that determine proton conductivity in nonstoichiometric complex perovskites. Solid State Ionics 125:303–311. doi:10.1016/S0167-2738(99)00189-7
Nowick AS, Du Y, Liang KC (2000) Effect of non-stoichiometry on the protonic and oxygen-ionic conductivity of Sr2(ScNb)O6: a complex perovskite. Solid State Ionics 129:201–207. doi:10.1016/S0167-2738(99)00326-4
Du Y, Nowick AS (1995) Structural transitions and proton conduction in nonstoichiometric A3BʹB2ʺO9 perovskite-type oxides. J Am Ceram Soc 78:3033–3039. doi:10.1111/j.1151-2916.1995.tb09079.x
Viana HDAL, Irvine JTS (2010) Characterisation of lower temperature sintered zinc-doped barium calcium niobate proton conducting electrolytes. J Mater Chem 20:8506–8511. doi:10.1039/C0JM01230K
Bhella SS, Thangadurai V (2009) Synthesis and characterization of carbon dioxide and boiling water stable proton conducting double perovskite-type metal oxides. J Power Sources 186:311–319. doi:10.1016/j.jpowsour.2008.09.110
Kan WH, Roushanafshar M, Vincent A, Fürstenhaupt T, Parvez M, Luo J, Thangadurai V (2013) Substitution of B-sites by Mn, Fe and Co in double perovskite-type Ba3CaNb2O9 on crystal structure and electrical properties. RSC Adv 3:23824–23832. doi:10.1039/c3ra44429e
Kan WH, Trang TT, Fürstenhaupt T, Thangadurai V (2011) Synthesis, rietveld refinement of crystal structure, electron diffraction, and electrical transport properties of Ba2(Ca1–x–yFexNby)(Nb1–zFez)O6–δ double perovskites. Can J Chem 89:688–696. doi:10.1139/v11-058
Trinh TT, Thangadurai V (2010) Effect of Ti substitution for Nb in double perovskite-type Ba3CaNb2O9 on chemical stability and electrical conductivity. Electrochim Acta 56:227–234. doi:10.1016/j.electacta.2010.08.094
Kan WH, Chen M, Bae J-S, Kim B-H, Thangadurai V (2014) Determination of Fe oxidation states in the B-site ordered perovskite-type Ba2Ca0.67Fe0.33NbO6-δ at the surface (nano-scale) and bulk by variable temperature XPS and TGA and their impact on electrochemical catalysis. J Mater Chem A 2(23):8736–8741. doi:10.1039/C4TA00811A
Kan WH, Lussier J, Bieringer M, Thangadurai V (2014) Studies on polymorphic sequence during the formation of the 1:1 ordered perovskite-type BaCa0.335 M0.165Nb0.5O3-δ (M = Mn, Fe, Co) using in situ and ex situ powder x-ray diffraction. Inorg Chem 53(19):10085–10093. doi:10.1021/ic501270k
Gerlach RG, Bhella SS, Thangadurai V (2009) Facile conversion of layered Ruddlesden-Popper-related structure Y2O3-doped Sr2CeO4 into fast oxide ion-conducting fluorite-type Y2O3-doped CeO2. Inorg Chem 48(1):257–266. doi:10.1021/ic801729x
Danielson E, Devenney M, Giaquinta DM, Golden JH, Haushalter RC, McFarland EW, Poojary DM, Reaves CM, Weinberg WH, Wu XD (1998) A rare-earth phosphor containing one-dimensional chains identified through combinatorial methods. Science 279(5352):837–839. doi:10.1126/science.279.5352.837
Danielson E, Devenney M, Giaquinta DM, Golden JH, Haushalter RG, McFarland EW, Poojary DM, Reaves CM, Weinberg WH, Di Wu X (1998) X-ray powder structure of Sr2CeO4: a new luminescent material discovered by combinatorial chemistry. J Mol Struct 470(1–2):229–235. doi:10.1016/s0022-2860(98)00485-2
Kan WH, Thangadurai V (2012) Thermochemistry of Sr2Ce1–xPrxO4 (x = 0, 0.2, 0.5, 0.8, and 1): variable-temperature and -atmosphere in situ and ex situ powder x-ray diffraction studies and their physical properties. Inorg Chem 51:8973–8981. doi:10.1021/ic301071x
Li Q, Thangadurai V (2011) Novel Nd2WO6-type Sm2-xAxM1-yByO6-δ (A = Ca, Sr; M = Mo, W; B = Ce, Ni) mixed conductors. J Power Sources 196:169–178. doi:10.1016/j.jpowsour.2010.06.055
Tao S, Irvine JTS (2003) A redox-stable, efficient anode for solid-oxide fuel cells. Nat Mater 2:320. doi:10.1038/nmat871
Tao S, Irvine JTS (2006) Phase transition in perovskite oxide La0.75Sr0.25Cr0.5Mn0.5O3-δ observed by in situ high-temperature neutron powder diffraction. Chem Mater 18:5453–5460. doi:10.1021/cm061413n
Kan WH, Trang TT, Fürstenhaupt T, Thangadurai V (2011) Development of novel Fe-doped barium calcium niobates as promising mixed conductors for solid oxide fuel cells (SOFCs). ECS Trans 35:1259–1266. doi:10.1149/1.3570111
Zhou X, Yan N, Chuang KT, Luo J (2014) Progress in La-doped SrTiO3 (LST)-based anode materials for solid oxide fuel cells, RSC Adv 4:118–131. doi:10.1039/c3ra42666a
Souza S, Visco SJ, De Jonghe LC (1997) Reduced-temperature solid oxide fuel cell based on YSZ thin-film electrolyte, J. Electrochem. Soc., 144, L35-L37. doi:10.1149/1.1837484
Acknowledgments
This research was supported through funding by the NSERC solid oxide fuel cell Canada Strategic Research Network from the Natural Science and Engineering Research Council of Canada (NSERC) and other sponsors listed at www.sofccanada.com and the NSERC through a Discovery Grants. One of us (VT) thanks the Canada Foundation for Innovation (CFI) for support as Leaders Opportunity Fund to establish advanced laboratory for material characterization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kan, W.H., Thangadurai, V. Challenges and prospects of anodes for solid oxide fuel cells (SOFCs). Ionics 21, 301–318 (2015). https://doi.org/10.1007/s11581-014-1334-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1334-6