Abstract
Background
Collagenase clostridium histolyticum (CCH) is a Food and Drug Administration-approved treatment for adult patients with Dupuytren’s contracture with a palpable cord that has been shown efficacious and safe in clinical trials.
Methods
This paper summarizes the most common post-marketing clinical adverse event (AE) reports received by the manufacturer of CCH and sponsor of the US Biologics License Application (Auxilium Pharmaceuticals, Malvern, PA, USA) during the first 12 months after drug approval and commercialization in the USA.
Results
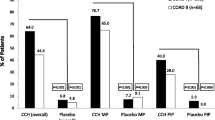
Of the 115 AE reports describing 270 AEs voluntarily received from patients or health care providers after approximately 5,400 injections of CCH administered, the most common AEs involved local, nonserious reactions to treatment, including skin tears, peripheral edema, and contusion. There were few serious AEs observed (0.6% reporting rate per 1,000 injections), and two flexor tendon ruptures and one flexor pulley injury were reported.
Conclusions
Analysis of post-marketing AEs received for CCH in the first year post-approval supports the safety profile reported earlier during clinical development and did not reveal additional clinical risks or concerns about CCH.
Similar content being viewed by others
References
Ahmad SR. Adverse drug event monitoring at the Food and Drug Administration. J Gen Intern Med. 2003;18:57–60.
Auxilium Pharmaceuticals Inc. AAC briefing document for collagenase clostridium histolyicum. Malvern, PA: Auxilium Pharmaceuticals, Inc.; September 16, 2009.
Chen NC, Srinivasan RC, Shauver MJ, Chung KC. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6:250–5.
Chigot PL. Foreword: Baron Dupuytren. In: Hueston JT, Tubiana R, editors. Dupuytren’s disease. New York: Grune & Stratton; 1974. ix–x.
Cooper A. A treatise on dislocations and fractures of the joints. London: Churchill; 1842.
Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. J Plast Surg. 2010;10:116–33.
Desai SS, Hentz VR. Collagenase clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10:1395–404.
Elliot D. The early history of contracture of the palmar fascia. Part 2: the revolution in Paris: Guillaume Dupuytren: Dupuytren’s disease. J Hand Surg Eur. 1988;13:371–8.
French MF, Mookhtiar KA, Van Wart HE. Limited proteolysis of type I collagen at hyperreactive sites by class I and II Clostridium histolyticum collagenases: complementary digestion patterns. Biochemistry. 1987;26:681–7.
Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N. Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am. 2010;35A:2027–2083.e1.
Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968–79.
Hutchison RL, Rayan GM. Astley Cooper: his life and surgical contributions. J Hand Surg Am. 2011;36:316–20.
Starkweather KD, Lattuga S, Hurst LC, Badalamente MA, Guilak F, Sampson SP, et al. Collagenase in the treatment of Dupuytren’s disease: an in vitro study. J Hand Surg Am. 1996;21:490–5.
Zhang AY, Curtin CM, Hentz VR. Flexor tendon rupture after collagenase injection for Dupuytren contracture: case report. J Hand Surg Am. 2011;36:1323–5.
Acknowledgments
The authors wish to thank Lynanne McGuire, PhD, of MedVal Scientific Information Services, LLC, for providing medical writing and editorial assistance. Funding for this study and support in the preparation of this manuscript was provided by Auxilium Pharmaceuticals. This manuscript was prepared according to the International Society for Medical Publication Professionals’ Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP2 Guidelines.
Conflict of interest
C. A. P. is a consultant for Auxilium Pharmaceuticals, from which he has received honoraria and reimbursement for travel and accommodation expenses. G. J. F’s employer, SSI Strategy, has received consulting fees or honoraria and travel and expenses reimbursement from Auxilium Pharmaceuticals, where he served as a safety consultant. C. A. M. is an employee of Auxilium Pharmaceuticals, where she is the Director of Drug Safety.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Peimer, C.A., McGoldrick, C.A. & Fiore, G.J. Nonsurgical treatment of Dupuytren’s contracture: 1-year US post-marketing safety data for collagenase clostridium histolyticum. HAND 7, 143–146 (2012). https://doi.org/10.1007/s11552-012-9407-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11552-012-9407-3