Abstract
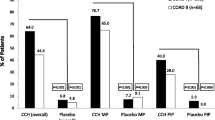
Collagenase Clostridium histolyticum (CCH; Xiapex®) is an injectable enzymatic collagenase approved for the treatment of Dupuytren’s contracture (DC). In two double-blind studies, up to three injections (mean of 1.5) of CCH per cord at monthly intervals were significantly more effective than placebo in reducing contractures, achieving clinical success in 64 % of treated joints affected by DC. CCH is generally well tolerated, with most adverse effects being local, transient injection-site reactions, such as oedema, pain and bruising. There is some evidence suggesting that CCH is associated with fewer major complications than open fasciectomy among selected patients. Estimated costs associated with CCH are generally lower than those associated with fasciectomy, but cost-effectiveness based on accepted willingness-to-pay thresholds is dependent on injection price.
Similar content being viewed by others
References
Azzopardi E, Boyce DE. Clostridium histolyticum collagenase in the treatment of Dupuytren’s contracture. Br J Hosp Med. 2012;73(8):432–6.
Schulze SM, Tursi JP. Postapproval clinical experience in the treatment of Dupuytren’s contracture with collagenase Clostridium histolyticum (CCH): the first 1000 days. Hand. 2014;9:447–58.
Mafi R, Hindocha S, Khan W. Recent surgical and medical advances in the treatment of Dupuytren’s disease: a systematic review of the literature. Open Orthop J. 2012;6(Suppl 1):77–82.
Needle or open fasciotomy for Dupuytren’s contracture: a review of the comparative efficacy, safety, and cost-effectiveness—an update. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2013.
Rodrigues JN, Becker GW, Ball C, et al. Surgery for Dupuytren’s contractures of the fingers. Cochrane Database Syst Rev. 2015;12:CD010143.
Xiapex (Collagenase Clostridium histolyticum): summary of product characteristics. London: European Medicines Agency; 2015.
Dhillon S. Collagenase Clostridium histolyticum: a review in Peyronie’s disease. Drugs. 2015;75(12):1405–12.
Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968–79.
Gilpin D, Coleman S, Hall S, et al. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am. 2010;35(12):2027–38 (e1).
Witthaut J, Jones G, Skrepnik N, et al. Efficacy and safety of collagenase Clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am. 2013;38(1):2–11.
Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40:1597–605.
Badalamente MA, Hurst LC, Benhaim P, et al. Efficacy and safety of collagenase Clostridium histolyticum in the treatment of proximal interphalangeal joints in Dupuytren contracture: combined analysis of 4 phase 3 clinical trials. J Hand Surg Am. 2015;40:975–83.
Bainbridge C, Gerber RA, Szczypa PP, et al. Efficacy of collagenase in patients who did and did not have previous hand surgery for Dupuytren’s contracture. J Plast Surg Hand Surg. 2012;46:177–83.
Warwick D, Arner M, Pajardi G, et al. Collagenase Clostridium histolyticum in patients with Dupuytren’s contracture: results from POINT X, an open-label study of clinical and patient-reported outcomes. J Hand Surg Eur. 2015;40(2):124–32.
Zhou C, Hovius SE, Slijper HP, et al. Collagenase Clostridium histolyticum versus limited fasciectomy for Dupuytren’s contracture: outcomes from a multicenter propensity score matched study. Plast Reconstr Surg. 2015;136(1):87–97.
Peimer CA, Wilbrand S, Gerber RA, et al. Safety and tolerability of collagenase Clostridium histolyticum and fasciectomy for Dupuytren’s contracture. J Hand Surg Eur. 2015;40(2):141–9.
Chen NC, Shauver MJ, Chung KC. Cost-effectiveness of open partial fasciectomy, needle aponeurotomy, and collagenase injection for Dupuytren contracture. J Hand Surg Am. 2011;36(1826–1834):e32.
Baltzer H, Binhammer PA. Cost-effectiveness in the management of Dupuytren’s contracture: a Canadian cost-utility analysis of current and future management strategies. Bone Joint J. 2013;95-B:1094–100.
De Salas-Cansado M, Cuadros M, Del Cerro M, et al. Budget impact analysis in Spanish patients with Dupuytren’s contracture: fasciectomy vs. collagenase Clostridium histolyticum. Chir Main. 2013;32(2):68–73.
Mehta S, Belcher HJ. A single-centre cost comparison analysis of collagenase injection versus surgical fasciectomy for Dupuytren’s contracture of the hand. J Plast Reconstr Aesthet Surg. 2014;67(3):368–72.
Sanjuan Cerveró R, Franco Ferrando N, Poquet Jornet J. Use of resources and costs associated with the treatment of Dupuytren’s contracture at an orthopedics and traumatology surgery department in Denia (Spain): collagenase Clostridium histolyticum versus subtotal fasciectomy. BMC Musculoskelet Disord. 2013;14:293.
Atroshi I, Strandberg E, Lauritzson A, et al. Costs for collagenase injections compared with fasciectomy in the treatment of Dupuytren’s contracture: a retrospective cohort study. BMJ Open. 2014;4(1):e004166.
Povlsen B, Povlsen SD. What is the better treatment for single digit Dupuytren’s contracture: surgical release or collagenase Clostridium histolyticum (Xiapex) injection? Hand Surg. 2014;19(3):389–92.
Nydick JA, Olliff BW, Garcia MJ, et al. A comparison of percutaneous needle fasciotomy and collagenase injection for Dupuytren disease. J Hand Surg Am. 2013;38:2377–80.
Coleman S, Gilpin D, Kaplan FT, et al. Efficacy and safety of concurrent collagenase Clostridium histolyticum injections for multiple Dupuytren contractures. J Hand Surg Am. 2014;39:57–64.
Gaston RG, Larsen SE, Pess GM, et al. The efficacy and safety of concurrent collagenase Clostridium histolyticum injections for 2 Dupuytren contractures in the same hand: a prospective, multicenter study. J Hand Surg Am. 2015;40:1963–71.
Acknowledgments
The review was reviewed by: F. Araujo, Pablo de Olavide University, Seville, Spain; S. Saluja, Saran Ashram Hospital, Dayalbagh, Agra, India; R. B. Shah, GMERS Medical College and Hospital, Gandhinagar, Gujarat, India; C. Zhou, Erasmus University Medical Centre, Rotterdam, The Netherlands. During the peer review process, the manufacturer of Xiapex® in Spain was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
K. McKeage and K. A. Lyseng-Williamson are salaried employees of Adis/Springer, are responsible for the article content and declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
McKeage, K., Lyseng-Williamson, K.A. Collagenase Clostridium histolyticum in Dupuytren’s contracture: a guide to its use in the EU. Drugs Ther Perspect 32, 131–137 (2016). https://doi.org/10.1007/s40267-016-0291-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-016-0291-8