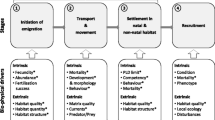
Abstract
In marine systems, adult populations confined to isolated habitat patches can be connected by larval dispersal. Source–sink theory provides effective tools to quantify the effect of specific habitat patches on the dynamics of connected populations. In this paper, we construct the next-generation matrix for a marine metapopulation and demonstrate how it can be used to calculate the source–sink dynamics of habitat patches. We investigate the effect of environmental variables on the source–sink dynamics and demonstrate how the next-generation matrix can provide useful biological insight into transient as well as asymptotic dynamics of the model.









Similar content being viewed by others
References
Aaen SM, Helgesen KO, Bakke MJ, Kaur K, Horsberg TE (2015) Drug resistance in sea lice: a threat to salmonid aquaculture. Trends Parasitol 31(2):72–81
Adams TP, Proud R, Black KD (2015) Connected networks of sea lice populations: dynamics and implications for control. Aquac Environ Interact 6(3):273–284
Aldrin M, Huseby RB, Stien A, Grøntvedt RN, Viljugrein H, Jansen PA (2017) A stage-structured Bayesian hierarchical model for salmon lice populations at individual salmon farms - Estimated from multiple farm data sets. Ecol Modell 359:333–348
Alexander SE, Roughgarden J (1996) Larval transport and population dynamics of intertidal barnacles: a coupled benthic/oceanic model. Ecol Monogr 66(3):259–275
Amarasekare P, Nisbet RM (2001) Spatial heterogeneity, source–sink dynamics, and the local coexistence of competing species. Am Nat 158(6):572–584
Armsworth PR (2002) Recruitment limitation, population regulation, and larval connectivity in reef fish metapopulations. Ecology 83(4):1092–1104
Bateman AW, Peacock SJ, Connors B, Polk Z, Berg D, Krkošek M, Morton A (2016) Recent failure to control sea louse outbreaks on salmon in the Broughton Archipelago, British Columbia. Can J Fish Aquat Sci 73(8):1164–1172
Botsford LW, Moloney CL, Hastings A, Largier JL, Powell TM, Higgins K, Quinn JF (1994) The influence of spatially and temporally varying oceanographic conditions on meroplanktonic metapopulations. Deep Res II 41(1):107–145
Brooks KM (2005) The effects of water temperature, salinity, and currents on the survival and distribution of the infective copepodid stage of sea lice (Lepeophtheirus salmonis) originating on atlantic salmon farms in the Broughton Archipelago of British Columbia, Canada. Rev Fish Sci 13(3):177–204
Cantrell DL, Rees EE, Vanderstichel R, Grant J, Filgueira R, Revie CW (2018) The use of kernel density estimation with a bio-physical model provides a method to quantify connectivity among Salmon farms: spatial planning and management with epidemiological relevance. Front Vet Sci 5(269):1–14
Chen C, Beardsley R, Cowles G (2006) An unstructured grid, finite-volume coastal ocean model (FVCOM) system. Oceanography 19(1):78–89
Costello MJ (2006) Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol 22(10):475–483
Costello MJ (2009) The global economic cost of sea lice to the salmonid farming industry. J Fish Dis 32(1):115–118
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Ann Rev Mar Sci 1:443–466
Cowen RK, Lwiza KM, Sponaugle S, Paris CB, Olson DB (2000) Connectivity of marine populations: open or closed? Science 287(5454):857–859
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of connectivity in marine populations. Science 311(5760):522–527
Diekmann O, Heesterbeek JAP, Metz JAJ (1990) On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J Math Biol 28(4):365–382
Diekmann O, Heesterbeek JAP, Roberts MG (2010) The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface 7(47):873–885
Feng Z, Thieme HR (2000) Endemic models with arbitrarily distributed periods of infection I: fundamental properties of the model. SIAM J Appl Math 61(3):803–833
Feng Z, Xu D, Zhao H (2007) Epidemiological models with non-exponentially distributed disease stages and applications to disease control. Bull Math Biol 69(5):1511–1536
Figueira WF, Crowder LB (2006) Defining patch contribution in source-sink metapopulations: the importance of including dispersal and its relevance to marine systems. Popul Ecol 48(3):215–224
Foreman MGG, Czajko P, Stucchi DJ, Guo M (2009) A finite volume model simulation for the Broughton Archipelago, Canada. Ocean Model 30(1):29–47
Godwin SC, Krkošek M, Reynolds JD, Rogers LA, Dill LM (2017) Heavy sea louse infection is associated with decreased stomach fullness in wild juvenile sockeye salmon. Can J Fish Aquat Sci 75(10):1587–1595
Gurtin ME, MacCamy RC (1974) Non-linear age-dependent population dynamics. Arch Ration Mech Anal 54(3):66–76
Gyllenberg M, Hanski I (1997) Habitat deterioration, habitat destruction, and metapopulation persistence in a heterogenous landscape. Theor Popul Biol 52(3):198–215
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49
Hastings A (1982) Dynamics of a single species in a spatially varying environment: the stabilizing role of high dispersal rates. J Math Biol 16(1):49–55
Heesterbeek JA, Roberts MG (2007) The type-reproduction number T in models for infectious disease control. Math Biosci 206(1):3–10
Horn RA, Johnson CR (2012) Matrix analysis, 2nd edn. Cambridge University Press, New York
Huang C, Cao J, Wen F, Yang X (2016a) Stability analysis of SIR model with distributed delay on complex networks. PLoS ONE 11(8):1–23
Huang Q, Lewis MA (2015) Homing fidelity and reproductive rate for migratory populations. Theor Ecol 8(2):187–205
Huang Q, Jin Y, Lewis MA (2016b) \(R_0\) analysis of a Benthic-Drift model for a stream population. SIAM J Appl Dyn Syst 15(1):287–321
Hurford A, Cownden D, Day T (2010) Next-generation tools for evolutionary invasion analyses. J R Soc Interface 7(45):561–571
Johnson SC, Albright LJ (1991) Development, growth, and survival of lepeophtheirus salmonis (Copepoda: Caligidae) under laboratory conditions. J Mar Biol Assoc United Kingdom 71(2):425–436
Jones G, Almany G, Russ G, Sale P, Steneck R, van Oppen M, Willis B (2009) Larval retention and connectivity among populations of corals and reef fishes : history, advances and challenges. Coral Reefs 28:307–325
Keyfitz BL, Keyfitz N (1997) The McKendrick partial differential equation and its uses in epidemiology and population study. Math Comput Model 26(6):1–9
Krkošek M, Lewis MA (2010) An \(R_0\) theory for source-sink dynamics with application to Dreissena competition. Theor Ecol 3(1):25–43
Krkošek M, Lewis MA, Morton A, Frazer LN, Volpe JP (2006) Epizootics of wild fish induced by farm fish. Proc Natl Acad Sci USA 103(42):15506–15510
Krkošek M, Ford JS, Morton A, Lele S, Myers RA, Lewis MA (2007) Declining wild salmon populations in relation to parasites from farm salmon. Science 318(2):1772–1775
Krkošek M, Connors BM, Morton A, Lewis MA, Dill LM, Hilborn R (2011) Effects of parasites from salmon farms on productivity of wild salmon. Proc Natl Acad Sci U S A 108(35):14700–14704
Krkošek M, Bateman AW, Proboszcz S, Orr C (2012a) Dynamics of outbreak and control of salmon lice on two salmon farms in the Broughton Archipelago, British Columbia. Aquac Environ Interact 1(2):137–146
Krkošek M, Connors BM, Lewis MA, Poulin R (2012b) Allee effects may slow the spread of parasites in a coastal marine ecosystem. Am Nat 179(3):401–412
Levins R (1969) Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am 15(3):237–250
Lewis MA, Shuai Z, van den Driessche P (2019) A general theory for target reproduction numbers with applications to ecology and epidemiology. J Math Biol 78(7):2317–2339
Li CK, Schneider H (2002) Applications of Perron-Frobenius theory to population dynamics. J Math Biol 44(5):450–462
McKendrick AG (1925) Applications of mathematics to medical problems. Proc Edinb Math Soc 44:98–130
McKenzie HW, Jin Y, Jacobsen J, Lewis MA (2012) \(R_0\) analysis of a spatiotemporal model for a stream population. SIAM J Appl Dyn Syst 11(2):567–596
Mileikovsky SA (1971) Types of larval development in marine bottom invertebrates, their distribution and ecological significance: a re-evaluation. Mar Biol Int J Life Ocean Coast Waters 10(3):193–213
Planes S, Jones GP, Thorrold SR (2009) Larval dispersal connects fish populations in a network of marine protected areas. Proc Natl Acad Sci 106(14):5693–5697
Pulliam HR (1988) Sources, sinks and population regulation. Am Nat 132(5):652–661
Revie CW, Robbins C, Gettinby G, Kelly L, Treasurer JW (2005) A mathematical model of the growth of sea lice, Lepeophtheirus salmonis, populations on farmed Atlantic salmon, Salmo salar L., in Scotland and its use in the assessment of treatment strategies. J Fish Dis 28(10):603–614
Roberts MG, Heesterbeek JA (2003) A new method for estimating the effort required to control an infectious disease. Proc R Soc B Biol Sci 270(1522):1359–1364
Rogers LA, Peacock SJ, McKenzie P, DeDominicis S, Jones SR, Chandler P, Foreman MG, Revie CW, Krkošek M (2013) Modeling parasite dynamics on farmed salmon for precautionary conservation management of wild salmon. PLoS ONE 8(4)
Roughgarden J, Gaines S, Possingham H (1988) Recruitment dynamics in complex life cycles. Science 241(4872):1460–1466
Stien A, Bjørn PA, Heuch PA, Elston DA (2005) Population dynamics of salmon lice Lepeophtheirus salmonis on Atlantic salmon and sea trout. Mar Ecol Prog Ser 290:263–275
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180(1–2):29–48
Watson JR, Kendall BE, Siegel DA, Mitarai S (2012) Changing seascapes, stochastic connectivity, and marine metapopulation dynamics. Am Nat 180(1):99–112
Acknowledgements
The authors would like to thank the members of the Lewis Lab for many helpful discussions and suggestions. PDH gratefully acknowledges an NSERC CGS-M scholarship and Queen Elizabeth II scholarship, and MAL gratefully acknowledges an NSERC Discovery Grant and a Canada Research Chair. We thank an anonymous reviewer for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Derivation from McKendrick–von Foerster PDE
Derivation from McKendrick–von Foerster PDE
Here, we derive Eq. 1 by solving the McKendrick–von Foerster PDE:
First, for simplicity we drop the indexes k and i so that \(n_k^i(t,a)=n(t,a)\). Then, we solve this linear partial differential equation using the method of characteristics. The goal is to reduce the partial differential equation into an ordinary differential equation of one variable along certain characteristic curves in a and t. To do this, we parameterize \(a=a(s)\) and \(t=t(s)\), so that n(t(s), a(s)) is now a function of the single variable s. Differentiating n(t(s), a(s)) with respect to s:
Now we choose the characteristic curves a(s) and t(s) such that
Then, substituting Eq. 14 into Eq. 15 we arrive at the ordinary differential equation:
Solving for the characteristic curves, t(s) and a(s), we find
Then, solving for n(t(s), a(s)) in Eq. 16 we find:
Now we have two boundary conditions to impose, one at \(t=0\) and one at \(a=0\). Together, the two boundaries intersect all characteristic curves, and so Eq. 17 is the unique solution to Eq. 14 for all \(a\ge \), \(t\ge 0\). From the form of our characteristic equations for a(s) and t(s), it is clear that all characteristics are lines \(t=a+b\) in the a-t plane. The line \(t=a\) divides the a-t plane into two regions: \(t\le a\) and \(t>a\). Characteristic curves for which \(t\le a\) intersect the boundary \(t=0\) at some point \((t,a)=(0,a_0)\). Substituting \(t=s\) and \(a=s+a_0\) into Eq. 17, we find
Similarly, characteristic curves for which \(t>a\) intersect the \(a=0\) boundary at some point \((t,a)=(t_0,0)\). Substituting \(t=s+t_0\) and \(a=s\) into Eq. 17, we find
Therefore, together we have
Rights and permissions
About this article
Cite this article
Harrington, P.D., Lewis, M.A. A Next-Generation Approach to Calculate Source–Sink Dynamics in Marine Metapopulations. Bull Math Biol 82, 9 (2020). https://doi.org/10.1007/s11538-019-00674-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-019-00674-1