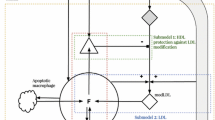
Abstract
Atherosclerosis is a major burden for all societies, and there is a great need for a deeper understanding of involved key inflammatory, immunological and biomechanical processes. A decisive step for the prevention and medical treatment of atherosclerosis is to predict what conditions determine whether early atherosclerotic plaques continue to grow, stagnate or become regressive. The driving biological and mechanobiological mechanisms that determine the stability of plaques are yet not fully understood. We develop a spatially resolved and quantitative mathematical model of key contributors of early atherosclerosis. The stability of atherosclerotic model plaques is assessed to identify and classify progression-prone and progression-resistant atherosclerotic regions based on measurable or computable in vivo inputs, such as blood cholesterol concentrations and wall shear stresses. The model combines Darcy’s law for the transmural flow through vessels walls, the Kedem–Katchalsky equations for endothelial fluxes of lipoproteins, a quantitative model of early plaque formation from a recent publication and a novel submodel for macrophage recruitment. The parameterization and analysis of the model suggest that the advective flux of lipoproteins through the endothelium is decisive, while the influence of the advective transport within the artery wall is negligible. Further, regions in arteries with an approximate wall shear stress exposure below 20% of the average exposure and their surroundings are potential regions where progression-prone atherosclerotic plaques develop.









Similar content being viewed by others
References
Ai L, Vafai K (2006) A coupling model for macromolecule transport in a stenosed arterial wall. Int J Heat Mass Transf 49(9):1568–1591
Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA (1995) The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol 128(6):1243–1253
Bear J, Bachmat Y (2012) Introduction to modeling of transport phenomena in porous media, vol 4. Springer, Berlin
Berliner J, Territo M, Sevanian A, Ramin S, Kim JA, Bamshad B, Esterson M, Fogelman AM (1990) Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Investig 85(4):1260
Bratzler RL, Chisolm GM, Colton CK, Smith KA, Lees RS (1977) The distribution of labeled low-density lipoproteins across the rabbit thoracic aorta in vivo. Atherosclerosis 28(3):289–307
Brown AJ, Teng Z, Evans PC, Gillard JH, Samady H, Bennett MR (2016) Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat Rev Cardiol 13:210–220
Brown MS, Goldstein JL, Krieger M, Ho Y, Anderson R (1979) Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol 82(3):597–613
Brown MS, Ho Y, Goldstein J (1980) The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem 255(19):9344–9352
Bulelzai MA, Dubbeldam JL (2012) Long time evolution of atherosclerotic plaques. J Theor Biol 297:1–10
Calvez V, Houot JG, Meunier N, Raoult A, Rusnakova G (2010) Mathematical and numerical modeling of early atherosclerotic lesions. ESAIM Proc Surv 30:1–14
Chalmers AD, Bursill CA, Myerscough MR (2017) Nonlinear dynamics of early atherosclerotic plaque formation may determine the efficacy of high density lipoproteins (hdl) in plaque regression. PLoS ONE 12(11):e0187,674
Chalmers AD, Cohen A, Bursill CA, Myerscough MR (2015) Bifurcation and dynamics in a mathematical model of early atherosclerosis. J Math Biol 71(6–7):1451–1480
Chang YS, Yaccino JA, Lakshminarayanan S, Frangos JA, Tarbell JM (2000) Shear-induced increase in hydraulic conductivity in endothelial cells is mediated by a nitric oxide-dependent mechanism. Arterioscler Thromb Vasc Biol 20(1):35–42
Cheng C, Helderman F, Tempel D, Segers D, Hierck B, Poelmann R, van Tol A, Duncker DJ, Robbers-Visser D, Ursem NT et al (2007) Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis 195(2):225–235
Cilla M, Peña E, Martínez MA (2014) Mathematical modelling of atheroma plaque formation and development in coronary arteries. J R Soc Interface 11(90):20130,866
Cobbold C, Sherratt J, Maxwell S (2002) Lipoprotein oxidation and its significance for atherosclerosis: a mathematical approach. Bull Math Biol 64(1):65–95
Cominacini L, Garbin U, Pasini AF, Davoli A, Campagnola M, Contessi GB, Pastorino AM, Cascio VL (1997) Antioxidants inhibit the expression of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radical Biol Med 22(1):117–127
Coussy O (2004) Poromechanics. Wiley, Chichester
Crosetto P, Reymond P, Deparis S, Kontaxakis D, Stergiopulos N, Quarteroni A (2011) Fluid-structure interaction simulation of aortic blood flow. Comput Fluids 43(1):46–57
Curmi PA, Juan L, Tedgui A (1990) Effect of transmural pressure on low density lipoprotein and albumin transport and distribution across the intact arterial wall. Circ Res 66(6):1692–1702
Cybulsky MI, Gimbrone MA Jr (1991) Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251(4995):788
Donea J, Huerta A (2003) Finite element methods for flow problems. Wiley, Chichester
El-Bassiouni E, Helmy M, El-Zoghby S, Kamel EN, Hosny R (2007) Relationship between level of circulating modified LDL and the extent of coronary artery disease in type 2 diabetic patients. Br J Biomed Sci 64(3):109–116
Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP et al (2004) Atherosclerotic vascular disease conference writing group III: Pathophysiology. Circulation 109(21):2617–2625
Feintuch A, Ruengsakulrach P, Lin A, Zhang J, Zhou YQ, Bishop J, Davidson L, Courtman D, Foster FS, Steinman DA et al (2007) Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am J Physiol Heart Circ Physiol 292(2):H884–H892
Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA et al (2003) The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5(9):781–792
Figueroa CA, Baek S, Taylor CA, Humphrey JD (2009) A computational framework for fluid-solid-growth modeling in cardiovascular simulations. Comput Methods Appl Mech Eng 198(45–46):3583–3602
Fok PW (2016) Multi-layer mechanical model of Glagov remodeling in coronary arteries: differences between in-vivo and ex-vivo measurements. PLoS ONE 11(7):e0159,304
Formaggia L, Quarteroni A, Veneziani A (2010) Cardiovascular mathematics: modeling and simulation of the circulatory system, vol 1. Springer, Berlin
Friedman A, Hao W (2015) A mathematical model of atherosclerosis with reverse cholesterol transport and associated risk factors. Bull Math Biol 77(5):758–781
Frostegard J, Haegerstrand A, Gidlund M, Nilsson J (1991) Biologically modified LDL increases the adhesive properties of endothelial cells. Atherosclerosis 90(2–3):119–126
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5(12):953–964
Hansson GK, Libby P (2006) The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6(7):508–519
Hao W, Friedman A (2014) The LDL-HDL profile determines the risk of atherosclerosis: a mathematical model. PLoS ONE 9(3):e90,497
Henriksen T, Mahoney EM, Steinberg D (1983) Enhanced macrophage degradation of biologically modified low density lipoprotein. Arterioscler Thromb Vasc Biol 3(2):149–159
Herrmann RA, Malinauskas RA, Truskey GA (1994) Characterization of sites with elevated LDL permeability at intercostal, celiac, and iliac branches of the normal rabbit aorta. Arterioscler Thromb Vasc Biol 14(2):313–323
Hoffman AH, Teng Z, Zheng J, Wu Z, Woodard PK, Billiar KL, Wang L, Tang D (2017) Stiffness properties of adventitia, media, and full thickness human atherosclerotic carotid arteries in the axial and circumferential directions. J Biomech Eng 139(12):124,501
Holzapfel GA, Sommer G, Gasser CT, Regitnig P (2005) Determination of layer-specific mechanical properties of human coronary arteries with nonatherosclerotic intimal thickening and related constitutive modeling. Am J Physiol Heart Circ Physiol 289(5):H2048–H2058
Hsiai TK, Cho SK, Wong PK, Ing M, Salazar A, Sevanian A, Navab M, Demer LL, Ho CM (2003) Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB J 17(12):1648–1657
Huang Z, Tarbell J (1997) Numerical simulation of mass transfer in porous media of blood vessel walls. Am J Physiol Heart Circ Physiol 273(1):H464–H477
Huo Y, Hafezi-Moghadam A, Ley K (2000) Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res 87(2):153–159
Jeng JR, Chang CH, Shih-Ming S, Hui-Chong C (1993) Oxidized low-density lipoprotein enhances monocyte-endothelial cell binding against shear-stress-induced detachment. Biochim Biophys Acta (BBA) Mol Cell Res 1178(2):221–227
Karner G, Perktold K (2000) Effect of endothelial injury and increased blood pressure on albumin accumulation in the arterial wall: a numerical study. J Biomech 33(6):709–715
Karner G, Perktold K, Zehentner HP (2001) Computational modeling of macromolecule transport in the arterial wall. Comput Methods Biomech Biomed Eng 4(6):491–504
Kedem O, Katchalsky A (1958) Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta 27:229–246
Kontush A, Chapman MJ (2006) Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev 58(3):342–374
Koshiba N, Ando J, Chen X, Hisada T (2007) Multiphysics simulation of blood flow and LDL transport in a porohyperelastic arterial wall model. J Biomech Eng 129(3):374–385
Ku DN, Giddens DP, Zarins CK, Glagov S (1985) Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arterioscler Thromb Vasc Biol 5(3):293–302
Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW (2002) Scavenger receptors class AI/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem 277(51):49,982–49,988
Lasseux D, Ahmadi A, Arani AAA (2008) Two-phase inertial flow in homogeneous porous media: a theoretical derivation of a macroscopic model. Transp Porous Media 75(3):371–400
Leake DS, Rankin SM (1990) The oxidative modification of low-density lipoproteins by macrophages. Biochem J 270(3):741–748
Lee YT, Lin HY, Chan YWF, Li KHC, To OTL, Yan BP, Liu T, Li G, Wong WT, Keung W et al (2017) Mouse models of atherosclerosis: a historical perspective and recent advances. Lipids Health and Dis 16(1):12
Lehoux S, Lévy BI (2006) Collateral artery growth. Circ Res 99:567–569
Lehr H, Hübner C, Nolte D, Finckh B, Beisiegel U, Kohlschütter A, Messmer K (1991) Oxidatively modified human low-density lipoprotein stimulates leukocyte adherence to the microvascular endothelium in vivo. Res Exp Med 191(1):85–90
Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7(9):678–689
Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874
Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z et al (2013) A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep 3(1):200–210
Liu X, Fan Y, Deng X (2010) Effect of spiral flow on the transport of oxygen in the aorta: a numerical study. Ann Biomed Eng 38(3):917–926
Mackness M, Abbott C, Arrol S, Durrington P (1993) The role of high-density lipoprotein and lipid-soluble antioxidant vitamins in inhibiting low-density lipoprotein oxidation. Biochem J 294(3):829–834
Maiellaro K, Taylor WR (2007) The role of the adventitia in vascular inflammation. Cardiovasc Res 75(4):640–648
Maoz H, Polliack A, Barak V, Yatziv S, Biran S, Giloh H, Treves AJ (1986) Parameters affecting the in vitro maturation of human monocytes to macrophages. Stem Cells 4(3):167–185
Melmed R, Karanian P, Berlin R (1981) Control of cell volume in the J774 macrophage by microtubule disassembly and cyclic AMP. J Cell Biol 90(3):761–768
Meyer G, Tedgui A et al (1996) Effects of pressure-induced stretch and convection on low-density lipoprotein and albumin uptake in the rabbit aortic wall. Circ Res 79(3):532–540
Moireau P, Xiao N, Astorino M, Figueroa CA, Chapelle D, Taylor CA, Gerbeau JF (2012) External tissue support and fluid–structure simulation in blood flows. Biomech Model Mechanobiol 11(1–2):1–18
Moore KJ, Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145(3):341–355
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ et al (2016) Heart disease and stroke statistics—2016 update. Circulation 133(4):e38–e360
Nagano Y, Arai H, Kita T (1991) High density lipoprotein loses its effect to stimulate efflux of cholesterol from foam cells after oxidative modification. Proc Natl Acad Sci 88(15):6457–6461
National Institutes of Health and National Heart, Lung and Blood Institute (2001) ATP III guidelines at-a-glance quick desk reference. NIH publication (01-3305)
Olgac U, Kurtcuoglu V, Poulikakos D (2008) Computational modeling of coupled blood-wall mass transport of LDL: effects of local wall shear stress. Am J Physiol Heart Circ Physiol 294(2):H909–H919
Olgac U, Poulikakos D, Saur SC, Alkadhi H, Kurtcuoglu V (2009) Patient-specific three-dimensional simulation of LDL accumulation in a human left coronary artery in its healthy and atherosclerotic states. Am J Physiol Heart Circ Physiol 296(6):H1969–H1982
Parton A, McGilligan V, O’Kane M, Baldrick FR, Watterson S (2016) Computational modelling of atherosclerosis. Brief Bioinform 17(4):562–575
Peiffer V, Sherwin SJ, Weinberg PD (2013) Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc Res 99(2):242–250
Phillips MC, Gillotte KL, Haynes MP, Johnson WJ, Lund-Katz S, Rothblat GH (1998) Mechanisms of high density lipoprotein-mediated efflux of cholesterol from cell plasma membranes. Atherosclerosis 137:S13–S17
Prosi M, Zunino P, Perktold K, Quarteroni A (2005) Mathematical and numerical models for transfer of low-density lipoproteins through the arterial walls: a new methodology for the model set up with applications to the study of disturbed lumenal flow. J Biomech 38(4):903–917
Rajman I, Eacho PI, Chowienczyk P, Ritter J (1999) LDL particle size: an important drug target? Br J Clin Pharmacol 48(2):125–133
Rappitsch G, Perktold K (1996) Pulsatile albumin transport in large arteries: a numerical simulation study. J Biomech Eng 118:511–519
Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LCM, Wofovitz E (2003) Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol 81(3):177–199
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340(2):115–126
Sherman TF (1981) On connecting large vessels to small. The meaning of Murray’s law. J Gen Physiol 78(4):431–453
Shirai T, Hilhorst M, Harrison DG, Goronzy JJ, Weyand CM (2015) Macrophages in vascular inflammation-from atherosclerosis to vasculitis. Autoimmunity 48(3):139–151
Sill HW, Chang YS, Artman JR, Frangos J, Hollis T, Tarbell J (1995) Shear stress increases hydraulic conductivity of cultured endothelial monolayers. Am J Physiol Heart Circ Physiol 268(2):H535–H543
Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation 92(5):1355–1374
Stender S, Zilversmit D (1981) Transfer of plasma lipoprotein components and of plasma proteins into aortas of cholesterol-fed rabbits. Molecular size as a determinant of plasma lipoprotein influx. Arterioscler Thromb Vasc Biol 1(1):38–49
Stocker R, Keaney JF (2004) Role of oxidative modifications in atherosclerosis. Physiol Rev 84(4):1381–1478
Sun N, Wood NB, Hughes AD, Thom SA, Xu XY (2006) Fluid-wall modelling of mass transfer in an axisymmetric stenosis: effects of shear-dependent transport properties. Ann Biomed Eng 34(7):1119–1128
Sun N, Wood NB, Hughes AD, Thom SAM, Xu XY (2007) Influence of pulsatile flow on LDL transport in the arterial wall. Ann Biomed Eng 35(10):1782–1790
Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP (2007) Hemodynamic shear stresses in mouse aortas implications for atherogenesis. Arterioscler Thromb Vasc Biol 27(2):346–351
Tabas I, Marathe S, Keesler GA, Beatini N, Shiratori Y (1996) Evidence that the initial up-regulation of phosphatidylcholine biosynthesis in free cholesterol-loaded macrophages is an adaptive response that prevents cholesterol-induced cellular necrosis proposed role of an eventual failure of this response in foam cell necrosis in advanced atherosclerosis. J Biol Chem 271(37):22773–22781
Tedgui A, Lever M (1984) Filtration through damaged and undamaged rabbit thoracic aorta. Am J Physiol Heart Circ Physiol 247(5):H784–H791
Thomas SR, Mikulecky DC (1978) Transcapillary solute exchange: a comparison of the Kedem–Katchalsky convection–diffusion equations with the rigorous nonlinear equations for this special case. Microvasc Res 15(2):207–220
Thon MP (2018) Multiphysics modeling and quantification of early atherosclerosis. Ph.D. thesis, Technische Universität München
Thon MP, Ford HZ, Gee MW, Myerscough MR (2018) A quantitative model of early atherosclerotic plaques parameterized using in vitro experiments. Bull Math Biol 80(1):175–214
Thon MP, Hemmler A, Glinzer A, Mayr M, Wildgruber M, Zernecke-Madsen A, Gee MW (2018) A multiphysics approach for modeling early atherosclerosis. Biomech Model Mechanobiol 17(3):617–644
Tomaso GD, Díaz-Zuccarini V, Pichardo-Almarza C (2011) A multiscale model of atherosclerotic plaque formation at its early stage. IEEE Trans Biomed Eng 58(12):3460–3463
Tompkins RG (1991) Quantitative analysis of blood vessel permeability of squirrel monkeys. Am J Physiol Heart Circ Physiol 260(4):H1194–H1204
Véniant MM, Withycombe S, Young SG (2001) Lipoprotein size and atherosclerosis susceptibility in Apoe-/- and Ldlr-/- mice. Arterioscler Thromb Vasc Biol 21(10):1567–1570
Wentzel JJ, Chatzizisis YS, Gijsen FJ, Giannoglou GD, Feldman CL, Stone PH (2012) Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: current understanding and remaining questions. Cardiovasc Res 96(2):234–243
Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG (2004) Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol 286(6):H2408–H2415
Whitman SC (2004) A practical approach to using mice in atherosclerosis research. Clin Biochem Rev 25(1):81
Williams KJ, Tabas I (1995) The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 15(5):551–561
Witztum JL, Steinberg D (1991) Role of oxidized low density lipoprotein in atherogenesis. J Clin Investig 88(6):1785
World Health Organisation (WHO) (2017) Cardiovascular diseases, Fact Sheet No. 317
Yang N, Vafai K (2006) Modeling of low-density lipoprotein (LDL) transport in the artery—effects of hypertension. Int J Heat Mass Transf 49(5):850–867
Yao PM, Tabas I (2000) Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem 275(31):23,807–23,813
Zi Z (2011) Sensitivity analysis approaches applied to systems biology models. IET Syst Biol 5(6):336–346
Acknowledgements
Michael W. Gee and Moritz P. Thon gratefully acknowledge the support and funding given by the International Graduate School of Science and Engineering of the TUM under project BioMat01, A Multiscale Model of Atherosclerosis and the Leibniz Rechenzentrum München of the Bavarian Academy of Sciences under contract number pr48ta. Mary R. Myerscough acknowledges support from an Australian Research Council Discovery Project Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that no conflicts of interest exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A Quantification of Model Parameters
In this section, a complete set of parameters for the spatially resolved model in SI units \(\mathrm {mm}\), \(\mathrm {g}\) and \(\mathrm {h}\) is derived. Due to a lack of uniform experimental data, we do not distinguish between experimental results gained from the study of different animal models. As the media is the predominant part of the intima–media domain (Fok 2016; Hoffman et al. 2017; Yang and Vafai 2006; Prosi et al. 2005), we use parameters that correspond to the media.
1.1 Appendix A.1 Macrophage-Related Parameters
The parameters of the submodel of monocyte adhesion least-squares fitted to the experimental results by Jeng et al. (1993) are
where all values are given in the unit of the experiment and SI units. The conversion of \(k_{m}\) from \({\upmu } \mathrm {g}\; \mathrm {lipid \; protein}\) to SI units is performed using \(\rho _{10}=1.04 \cdot 10^{9}\; \frac{1}{{\upmu } \mathrm {g}\; \mathrm {lipid \; protein}}\) from Thon et al. (2018), Table 1. An overview of the least-squares fits is shown in Fig. 10a. Since intracellular free and esterified cholesterol can only diffuse within macrophages, the macrophages’ effective diffusion coefficient is also employed for the cholesterols, i.e., \(D_{\mathrm {Eff},{m}} =D_{\mathrm {Eff},{f}}=D_{\mathrm {Eff},{b}} =3.6 \cdot 10^{-6} \; \frac{\mathrm {mm}^2}{\mathrm {h}}\), cf. Cilla et al. (2014) and Friedman and Hao (2015).
Least-squares fits of a simulated monocyte adhesion \(m(T_\mathrm {Adh})\) to experimental results by Jeng et al. (1993), Table 1, b filtration reflection coefficient \(\sigma _{\mathrm {F},i}\) to results by Karner et al. (2001), Table 3, c hindrance coefficient \(K_i\) to result by Formaggia et al. (2010) and d effective diffusion coefficient \(D_{\mathrm {Eff},i}\) by Karner et al. (2001), Table 2 (Color figure online)
1.2 Appendix A.2 LDL-Related Parameters
The influx of native LDL through the endothelium described by the second Kedem–Katchalsky equation [cf. Eq. (4)] is well studied, and thus the required parameters are available in the literature. In contrast, the flux of modified LDL and native HDL is scant investigated. Due to its origin and equal size, it is convenient to use the same parameters for native and modified LDL. In contrast to native LDL, however, the concentration of modified LDL in blood is low (El-Bassiouni et al. 2007), and therefore we use \(\eta _{{{\tilde{\ell }}}}=0\).
The weighting factors \(\omega _{\ell }\), \(\omega _{{{\tilde{\ell }}}}\) and \(\omega _{h}\) for the average concentrations of native LDL, modified LDL and native HDL within the endothelium layer are estimated as proposed by Formaggia et al. (2010). The one-dimensional advection–diffusion equation is solved and volume-averaged over the domain of the endothelium. It follows that
where \(Pe_i\) is the Péclet number of species i within the endothelium given by
Here, \(\Vert {\varvec{u}} \Vert \) and \(P_i\) are the transmural filtration velocity and the diffusive permeability of species i, respectively. Using \(\Vert {{\varvec{u}} \Vert =6.41 \cdot 10^{-2} \; \frac{\mathrm {mm}}{\mathrm {h}}}\) (Formaggia et al. 2010; Yang and Vafai 2006; Meyer and Tedgui 1996) and \(P_{\ell }= P_{{{\tilde{\ell }}}}= 6.12 \cdot 10^{-5} \; \frac{\mathrm {mm}}{\mathrm {h}}\) (Tompkins 1991; Yang and Vafai 2006) results in \(Pe_{\ell }=Pe_{{{\tilde{\ell }}}}= 1.05 \cdot 10^{3}\) and weighting factors \(\omega _{\ell }=\omega _{{{\tilde{\ell }}}}=9.99 \cdot 10^{-1}\).
To estimate the initial rate of recruitment of macrophages \(r_{m}({{{\tilde{\ell }}}}_0,\Vert {\varvec{\tau }} \Vert )\), we use an initial concentration of modified LDL \({{{\tilde{\ell }}}}_0=7.2 \cdot 10^{-4} \; \eta _{\ell }\) based on the experimental results in Herrmann et al. (1994) and Tompkins (1991).
1.3 Appendix A.3 HDL-Related Parameters
HDL is a smaller particle than LDL. More precisely, LDL and HDL have radii of \({R_{\ell }=11.0 \; \mathrm {nm}}\) (Karner et al. 2001) and \({R_{h}=4.72 \; \mathrm {nm}}\) (Kontush and Chapman 2006), respectively. As a consequence, HDL has approximately an 1.87 times higher diffusive permeability compared to LDL, i.e., \(P_{h}=1.87 \; P_{\ell }=1.14 \cdot 10^{-4} \; \frac{\mathrm {mm}}{\mathrm {h}}\) (Stender and Zilversmit 1981; Tompkins 1991). Together with Eqs. (23) and (24), it follows the weighting factor \(\omega _{h}=9.98 \cdot 10^{-1}\) for the average concentrations of native HDL in the endothelium layer.
Karner et al. (2001) investigated the transport rates of ADP, albumin and LDL through the endothelium and the internal elastic lamina. As ADP and albumin have a smaller and LDL has a larger radius compared to HDL, their filtration reflection coefficients are used to estimate the filtration reflection coefficient \(\sigma _{\mathrm {F},{h}}\) of HDL. We performed a least-squares fit of the filtration reflection coefficient as given in Karner et al. (2001), Table 3 to a saturating function in the particle radius \(R_i\) of the form \(\sigma _{\mathrm {F},i}(R_i) = \frac{R_i^a}{b^a+R_i^a}\). The least-squares fit yields \(a=3.006\) and \(b=2.112 \; \mathrm {nm}\) and so
which is visualized in Fig. 10b. The filtration reflection coefficient scales approximately with the volume of particles, and for HDL this gives \({\sigma _{\mathrm {F},{h}}=9.18 \cdot 10^{-1}}\).
The hindrance coefficient of LDL in the media is given by \({K_{{\ell }}=K_{{{{\tilde{\ell }}}}}=0.117}\) (Formaggia et al. 2010; Olgac et al. 2009), respectively. Assuming that the hindrance coefficient scales with the volume of particles (in consistent with the filtration reflection coefficient) and a saturating kinetic (in general it holds \(0 \le K_i(R_i) \le 1\)), it follows
which is plotted in Fig. 10c. Accordingly, the hindrance coefficient of HDL is estimated to \(K_{h}=6.27 \cdot 10^{-1}\).
An additional unknown parameter is the effective diffusion coefficient of HDL \(D_{\mathrm {Eff},h}\) within the artery wall. The effective diffusivity of LDL is measured as \(D_{\mathrm {Eff},{\ell }}=D_{\mathrm {Eff},{{{\tilde{\ell }}}}}=1.26 \cdot 10^{-2} \; \frac{\mathrm {mm}^2}{\mathrm {h}}\) (Tomaso et al. 2011; Sun et al. 2007; Calvez et al. 2010), but no experimental results exist that allow quantifying the effective diffusivity of HDL. However, Karner et al. (2001) give the diffusivities of ADP, albumin and LDL in blood plasma. The data in Karner et al. (2001) are therefore normalized to the diffusivity of LDL in blood plasma and least-squares fitted to a rational function of the form \(\frac{D_{\mathrm {Eff},i}(R_i)}{D_{\mathrm {Eff},{\ell }}}=\frac{e}{R_i^f}\). The least-squares fit yields \(e=11.0 \; \mathrm {nm}\) and \(f=1.0\) such that
which is plotted in Fig. 10d. Here, the effective diffusion coefficients scales with the radius of particles and for HDL it yields \(D_{\mathrm {Eff},{h}}=2.33 \; D_{\mathrm {Eff},{\ell }}=2.94 \cdot 10^{-2} \; \frac{\mathrm {mm}^2}{\mathrm {h}}\).
The rates of oxidative modification of HDL by macrophages \(q_{{h},{m}}\) and endothelial cells \(q_{{h},{e}}\) are crucial, but no experimental results exist that allow us to quantify them directly. Hence, the rates of HDL modification are calculated from the rates of LDL modification by considering the different structure and size of LDL and HDL. Therefore, we use
where \(N_{{{\tilde{\ell }}}}\) and \(N_{{\tilde{h}}}\) correspond to the amount of lipid peroxide in modified LDL and HDL, respectively. The factor 7.6 represents the relative difference between the surface areas of LDL and HDL (Cobbold et al. 2002). Using the values of \(q_{{\ell },{m}}, q_{{\ell },{e}}, N_{{{\tilde{\ell }}}}\) and \(N_{{\tilde{h}}}\) given in Thon et al. (2018), Table 4 yields \(q_{{h},{m}}=1.64 \cdot 10^{-4} \; \frac{\mathrm {mm}^3}{\mathrm {h}}\) and \(q_{{h},{e}}=6.21 \cdot 10^{-2} \; \frac{\mathrm {mm}}{\mathrm {h}}\).
1.4 Appendix A.4 Transmural flow
The Darcy permeability K must be estimated to fit to the murine physiology, i.e., to the murine intima–media thickness. The Darcy permeability is approximated by solving the norm of Eq. (9) for K:
where \(\Vert {\varvec{u}} \Vert \) and \(\Vert \nabla p_\mathrm {Med} \Vert \) correspond to the transmural filtration velocity and pressure gradient in the intima and media, respectively. The latter is approximated by the luminal blood pressure \(\eta _p=100 \; \mathrm {mmHg}\) (Ai and Vafai 2006; Olgac et al. 2008; Whitesall et al. 2004), the pressure drop across the endothelium \({\varDelta p_\mathrm {End}=18 \; \mathrm {mmHg}}\) (Tedgui and Lever 1984) and the adventitial pressure \({p_\mathrm {Adv}=30 \; \mathrm {mmHg}}\) (Ai and Vafai 2006; Olgac et al. 2008; Yang and Vafai 2006) by: \({\Vert \nabla p_\mathrm {Med} \Vert = \frac{\eta _p - \varDelta p_\mathrm {End}-p_\mathrm {Adv}}{H}}=\frac{52 \; \mathrm {mmHg}}{0.04 \; \mathrm {mm}}\)\({=1.73 \cdot 10^{5} \; \frac{\mathrm {Pa}}{\mathrm {mm}}}\). Assuming a transmural filtration velocity \({\Vert {{\varvec{u}} \Vert =6.41 \cdot 10^{-2} \; \frac{\mathrm {mm}}{\mathrm {h}}}}\) (Formaggia et al. 2010; Yang and Vafai 2006; Meyer and Tedgui 1996), a porosity of \(\phi =0.15\) (Ai and Vafai 2006; Prosi et al. 2005) and a dynamic viscosity of blood plasma of \(\mu =2.0 \cdot 10^{-7} \;\mathrm {Pa}\; \mathrm {h}\) (Prosi et al. 2005; Yang and Vafai 2006) yield a Darcy permeability of \({K= 1.11 \cdot 10^{-14} \; \mathrm {mm}^2}\).
An overview of all parameters estimated and fitted so far is given in Table 2.
1.5 Appendix A.5 Wall Shear Stress-Dependent Parameters
There are experimental data by Sill et al. (1995) that quantify the hydraulic conductivity with respect to the WSS \(\Vert {\varvec{\tau }} \Vert \). Sun et al. (2006) used these experimental results to parameterize the CSF (cf. Eq. (7)) which leads to: \(\gamma _p=1.31 \cdot 10^{-1}\), \(\mu _p=1.24 \cdot 10^{3} \; \frac{1}{\mathrm {Pa}}\) and \(\xi _p=1.86 \cdot 10^{1}\). In Sun et al. (2006), the parameter \(\mu _p\) was scaled so that it fits to a reference WSS value of \(\Vert \overline{{\varvec{\tau }}}\Vert = 1.68 \; \mathrm {Pa}\). Even though the distribution of the reference WSS value \(\Vert \overline{{\varvec{\tau }}}\Vert \) within an animal is more or less uniform (Sherman 1981), it varies significantly in between different animal models (Cheng et al. 2007). Therefore, the parameter \(\mu _p\) of the CSF must be adapted to match the physiology of the animal under consideration, i.e., the murine physiology.
The same holds for the parameters \(\xi _\tau \) and \(\gamma _\tau \) of the macrophage recruitment submodel (cf. Eq. (3)) and the PSF (cf Eq. (6)). The estimated value \(\xi _\tau =1.95 \cdot 10^{-1} \;\mathrm {Pa}\) corresponds to the human physiology with a reference WSS value \(\Vert \overline{{\varvec{\tau }}}\Vert = 1.16 \; \mathrm {Pa}\) (Cheng et al. 2007). The parameter \(\gamma _\tau \) is linked to a given reference WSS value \(\Vert \overline{{\varvec{\tau }}}\Vert \) by \(\gamma _\tau = \frac{1}{30} \Vert \overline{{\varvec{\tau }}}\Vert \), cf. Thon et al. (2018). A tabular overview of values for \(\Vert \overline{{\varvec{\tau }}}\Vert \), \(\xi _\tau \), \(\gamma _\tau \) and \(\mu _p\) for different animal models and the normalized case is given in Table 3.
1.6 Appendix A.6 Plaque-Specific Parameters
It remains to specify the concentrations of native LDL \(\eta _{\ell }\) and HDL \(\eta _{h}\) in blood and the WSS \(\Vert {\varvec{\tau }} \Vert \) that the model plaque is exposed to in vivo. These parameters characterize the physiology and diet of the plaque’s host and the position of the plaque in the cardiovascular system. Thus, they cannot be set at fixed values, but their full ranges must be considered. The WSS \(\Vert {\varvec{\tau }} \Vert \) within a murine aortic arch is below approximately \(7.7 \Vert \overline{{\varvec{\tau }}}\Vert \) (Thon et al. 2018). In a clinical context, the concentrations of LDL and HDL are measured by determining their cholesterol contents in blood. In humans, the physiological ranges lie approximately in between concentrations of \(50 {-} 250 \; \frac{\mathrm {mg}}{\mathrm {dl}}\) of LDL blood cholesterol and \(20 {-} 80 \; \frac{\mathrm {mg}}{\mathrm {dl}}\) of HDL blood cholesterol (National Institutes of Health and National Heart, Lung and Blood Institute 2001), which can be transformed into SI units of particles per volume using \(\rho _{8}=4.36 \cdot 10^{17} \; \frac{1}{\mathrm {g}}\) and \(\rho _{13}=7.34 \cdot 10^{18} \; \frac{1}{\mathrm {g}}\) (see Thon et al. 2018, Table 1), respectively. In the case of mice with genetic modifications and high-fat diets, the LDL and HDL cholesterol concentrations can rise above \(3000 \; \frac{\mathrm {mg}}{\mathrm {dl}}\) and \(400 \; \frac{\mathrm {mg}}{\mathrm {dl}}\), respectively (Véniant et al. 2001; Lee et al. 2017). In general, however, mice show lower total blood cholesterol concentrations compared to humans, and in mice, as opposed to humans, HDL cholesterol is the predominant lipoprotein (Lee et al. 2017). An overview of the full spectrum of LDL and HDL blood cholesterol concentrations as well as WSS is given in Table 4.
Appendix B Non-spatial Model
A non-spatial version of the spatially resolved model developed in Sect. 2.2 is first given in this section. It is important to note that the non-spatial model is equivalent to the spatially resolved model, where the concentrations are homogeneous, e.g., induced by high effective diffusion coefficients \(D_{\mathrm {Eff},i}\rightarrow \infty \) (\(i={\ell },\ldots ,m\)). Subsequently, the non-spatial model is used to effectively perform sensitivity and stability analyses.
1.1 Appendix B.1 Model Overview and Proposition 1
The non-spatial model of early atherosclerosis consists of the parameterized ODE model developed in Thon et al. (2018) enriched by the submodels of macrophage recruitment and lipoprotein fluxes in Sects. 2.1.2 and 2.1.3 . Altogether, the non-spatial model reads
where the originally fixed-valued parameters corresponding to the fluxes of native LDL \(r_{\ell }\), modified LDL \(r_{{{\tilde{\ell }}}}\) and native HDL \(r_{h}\) as well as the recruitment of macrophages \(r_{m}\) were already notated including their explicit dependencies given by the submodels:
The initial conditions of the non-spatial model read analog to Thon et al. (2018)
where \(m_0\) is adapted due to the employed submodel of macrophage recruitment:
Proposition 1
Let \({\ell }(t),{{{\tilde{\ell }}}}(t),h(t),f(t),b(t),m(t) \ (t \ge 0)\) be the unique and smooth solution of the initial value problem defined by Eqs. (30)–(33) with strictly positive parameters fulfilling \( (1-\sigma _{\mathrm {F},i})(1-\omega _i)J_\mathrm {Vol}(p,\Vert {\varvec{\tau }} \Vert ) \le P_i s_\mathrm {P}(\Vert {\varvec{\tau }} \Vert )\), \(0 \le \omega _i \le 1\), \({0 \le \sigma _{\mathrm {F},i} \le 1} \ ({i={\ell },{{{\tilde{\ell }}}},h})\), \( 0 \le p \le \eta _p\), \(f_\mathrm {Min} \le f_\mathrm {In} < f_\mathrm {Max}\) and \(f_\mathrm {Min} \le f_0 < f_\mathrm {Max}\). Then, the solution satisfies:
-
1.
\({\ell }(t), {{{\tilde{\ell }}}}(t), h(t), f(t), b(t), m(t) \ge 0\) for all \(t \ge 0\).
-
2.

\(\frac{f(t)}{m(t)} \in \left[ f_\mathrm {Min} , f_\mathrm {Max} \right] \) for all \(t \ge 0\).
-
3.
\({\ell }(t), {{{\tilde{\ell }}}}(t), h(t), f(t), m(t)\) are bounded.
Proof
The proof of Proposition 1 is similar to the proof of the proposition given in Thon et al. (2018). First, it follows from \( \eta _p \ge p\) that
To prove the positivity of \({\ell }\), i.e., \({\ell }(t)\ge 0 \; \forall t\ge 0\), it is sufficient to note that \({\ell }(0)=0\) and that \({\ell }(t)=0\) implies
due to the strict positivity of the parameters. The positivities of \(h, m\) and \({{{\tilde{\ell }}}}\) are proved in an analog fashion. Since \(\frac{f(0)}{m(0)}=f_0 \ge f_\mathrm {Min}\) and \(\frac{f(t)}{m(t)}=f_\mathrm {Min}\) implies
it holds \(\frac{f(t)}{m(t)} \ge f_\mathrm {Min} \; \forall t \ge 0\). This also implies the positivity of \(f\). Given that \(\frac{f(0)}{m(0)}=f_0 < f_\mathrm {Max}\) and that \(\frac{f(t)}{m(t)}\rightarrow f_\mathrm {Max}\) implies
it follows \(\frac{f(t)}{m(t)} \le f_\mathrm {Max} \; \forall t \ge 0\). Hence, it is concluded that
The positivity of \(b\) follows since \(b(0)=0\) and \(b(t)=0\) implies
which finishes the proof of 1. (It also follows that the time-dependent solution \(({\ell }(t),{{{\tilde{\ell }}}}(t),h(t),\)\(f(t),b(t),m(t)), t\ge 0\) of the initial value problem is unique and smooth because the smoothness of the right-hand side of the ODE (30) is now straightforward to show.)
Using (38)
holds, and by solving this ordinary differential inequality with associated initial condition \({m(0)=m_0}\) it follows
In an analog manner, the upper bound for \(m(t)\) is found, leading to
which finishes the proof of 2.
Due to \( (1-\sigma _{\mathrm {F},{\ell }})(1-\omega _{\ell })J_\mathrm {Vol}(p,\Vert {\varvec{\tau }} \Vert ) \le P_{\ell }s_\mathrm {P}(\Vert {\varvec{\tau }} \Vert )\), the boundedness of \({\ell }(t)\) is given by
since the solution of the ordinary differential inequality (with associated initial condition \({\ell }(0)=0\)) is bounded by
In an analog manner, the boundedness of \({{{\tilde{\ell }}}}(t)\) and \(h(t)\) are proved which finishes the proof of Proposition 1. \(\square \)
1.2 Appendix B.2 Sensitivity Analysis
The parameters estimated in Appendix A have a degree of uncertainty due to their origins, indifferent pieces of research used as experimental models, and measurement errors. To quantify the effect of uncertainties in the parameters on the computational results of the model, a local sensitivity analysis using the method of a metabolic control analysis (Zi 2011) is performed. However, the computational cost to do this analysis for the spatially resolved model is too large, so that a metabolic control analysis using the spatially resolved model is not achievable. Therefore, we use the non-spatial model from Appendix B.1 with prescribed subendothelial pressure \(p=\eta _p-\varDelta p_\mathrm {End}= 82 \; \mathrm {mmHg}= 1.09 \cdot 10^4 \; \mathrm {Pa}\) (Tedgui and Lever 1984; Tomaso et al. 2011; Olgac et al. 2008; Cilla et al. 2014), LDL cholesterol concentration \({\eta _{\ell }=150 \; \frac{\mathrm {mg}}{\mathrm {dl}}}\), HDL cholesterol concentration \(\eta _{h}=50 \; \frac{\mathrm {mg}}{\mathrm {dl}}\) and WSS \(\Vert {\varvec{\tau }} \Vert = 10\% \Vert \overline{{\varvec{\tau }}}\Vert \).
The metabolic control analysis is performed in the same way as in Zi (2011) and Thon et al. (2018). Accordingly, we compare the normalized partial derivatives of all concentrations with respect to all non-spatial parameters p given in Table 2 at time \(T=100 \; \text {weeks}\). The uncertainties of the remaining non-spatial parameters were previously addressed in Thon et al. (2018). As proposed in Zi (2011), the partial derivatives are estimated by using forward finite difference approximations with a sufficiently small variation parameter \(\varepsilon =0.1\%\). Hence, the metabolic control coefficient \(\mathrm {MCC}({\ell },p)\) of LDL \({\ell }\) with respect to a model parameter p is computed by
where \({\ell }_{p+\varepsilon \%}\) denotes the concentration of native LDL \({\ell }\) computed with the parameter p perturbed by \(\varepsilon \)%. The metabolic control coefficients of all other species are computed in the same manner. The metabolic control coefficients of all species of the non-spatial model with respect to all non-spatial parameters from Table 2 are plotted in Fig. 11.
Sensitivity analysis of the non-spatial model. Metabolic control coefficients of native LDL \({\ell }\), modified LDL \({{{\tilde{\ell }}}}\), HDL \(h\), intracellular free cholesterol per macrophage \(\frac{f}{m}\), intracellular cholesterol ester per macrophage \(\frac{b}{m}\) and macrophages \(m\) with respect to all non-spatial parameters p in Table 2. The values of the truncated bars are \(\mathrm {MCC}({\ell },\sigma _{\mathrm {F},{\ell }})=-175.90\) and \(\mathrm {MCC}({{{\tilde{\ell }}}},\sigma _{\mathrm {F},{\ell }})=-92.68\) (Color figure online)
From the sensitivity analysis, it follows that variations of the filtration reflection coefficients of LDL \(\sigma _{\mathrm {F},{\ell }}\) and HDL \(\sigma _{\mathrm {F},{h}}\) have the largest influence on the computational results. While the impact of the filtration reflection coefficient of HDL \(\sigma _{\mathrm {F},{h}}\) is still moderate, the effect of slight deviation of \(\sigma _{\mathrm {F},{\ell }}\) is severe. Even though the literature in general agrees on \(\sigma _{\mathrm {F},{\ell }} \approx 9.97 \cdot 10^{-1}\) (Karner et al. 2001; Yang and Vafai 2006; Sun et al. 2007; Prosi et al. 2005; Ai and Vafai 2006), both parameters \(\sigma _{\mathrm {F},{\ell }}\) and \(\sigma _{\mathrm {F},{h}}\) require further attention, especially by experimental communities.
1.3 Appendix B.3 Stability Analysis
The outcome of the spatially resolved model is strongly dependent on blood cholesterol concentrations of LDL \(\eta _{\ell }\) and HDL \(\eta _{h}\) and the WSS \(\Vert {\varvec{\tau }} \Vert \) which characterize the physiology and diet of the host and the position of the individual plaque within the cardiovascular system. They can—depending on the species of host, its predisposition and its diet—vary by several orders of magnitude (cf. Table 4) which results in qualitatively different predicted long-term outcomes. Due to the high computational cost of the spatially resolved model, a stability analysis is performed using the non-spatial model from Appendix B.1.
Proposition 1 from Appendix B.1 applies, as all its requirements are fulfilled by the non-spatial model with the complete parameter set from Tables 2 and 3 , and Thon et al. (2018), Table 4. Hence, the steady-state concentrations of macrophages \({{\widehat{{m}}}}\) and intracellular free cholesterol per macrophage \(\frac{{{\widehat{f}}}}{{{\widehat{m}}}}\) satisfy
Stability analysis of the non-spatial model. Boundedness of intracellular cholesterol ester (a–d), steady-state concentration of intracellular free cholesterol per macrophage \(\displaystyle \frac{{{\widehat{f}}}}{{{\widehat{m}}}}\) (e–h), and steady-state density of macrophages \({{\widehat{m}}}\) (i–l) are predicted for varying LDL and HDL cholesterol concentrations \(\eta _{\ell }\) and \(\eta _{h}\) and various WSS \(\Vert {\varvec{\tau }} \Vert \) (Color figure online)

and only the concentration of cholesterol ester b can be unbounded. The boundedness of intracellular cholesterol ester b, the steady-state concentration of intracellular free cholesterol per macrophages \(\frac{{{\widehat{f}}}}{{{\widehat{m}}}}\) and the steady-state density of macrophages \({{\widehat{m}}}\) are computed in the crucial ranges of \(\eta _{\ell }\), \(\eta _{h}\) and \(\Vert {\varvec{\tau }}\Vert \). High LDL cholesterol, low HDL cholesterol and low WSS promote atherosclerosis, and so the ranges that we use for the stability analysis are \(\eta _{\ell }\in [10, 800 ] \; \frac{\mathrm {mg}}{\mathrm {dl}}\), \(\eta _{h}\in [20 , 100 ] \; \frac{\mathrm {mg}}{\mathrm {dl}}\) and \(\Vert {\varvec{\tau }} \Vert \in [5\% , 40\% ] \Vert \overline{{\varvec{\tau }}}\Vert \), cf. Table 4. The points where analysis is done are on an equidistant grid of the specified parameter space. The numerical results for varying blood cholesterol concentrations \(\eta _{\ell }\) and \(\eta _{h}\) and four different WSS levels \(\Vert {\varvec{\tau }} \Vert \) are plotted in Fig. 12.
Rights and permissions
About this article
Cite this article
Thon, M.P., Myerscough, M.R. & Gee, M.W. A Spatially Resolved and Quantitative Model of Early Atherosclerosis. Bull Math Biol 81, 4022–4068 (2019). https://doi.org/10.1007/s11538-019-00646-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-019-00646-5