Abstract
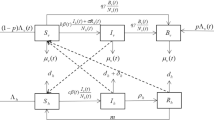
Recent experimental study suggests that the engineered symbiotic bacteria Serratia AS1 may provide a novel, effective and sustainable biocontrol of malaria. These recombinant bacteria have been shown to be able to rapidly disseminate throughout mosquito populations and to efficiently inhibit development of malaria parasites in mosquitoes in controlled laboratory experiments. In this paper, we develop a climate-based malaria model which involves both vertical and horizontal transmissions of the engineered Serratia AS1 bacteria in mosquito population. We show that the dynamics of the model system is totally determined by the vector reproduction ratio \(R_\mathrm{v}\), and the basic reproduction ratio \(R_0\). If \(R_\mathrm{v}\le 1\), then the mosquito-free state is globally attractive. If \(R_\mathrm{v}>1\) and \(R_0\le 1\), then the disease-free periodic solution is globally attractive. If \(R_\mathrm{v}>1\) and \(R_0>1\), then the positive periodic solution is globally attractive. Numerically, we verify the obtained analytic result and evaluate the effects of releasing the engineered Serratia AS1 bacteria in field by conducting a case study for Douala, Cameroon. We find that ideally, by using Serratia AS1 alone, it takes at least 25 years to eliminate malaria from Douala. This implies that continued long-term investment is needed in the fight against malaria and confirms the necessity of integrating multiple control measures.




Similar content being viewed by others
References
Ai S, Li J, Lu J (2012) Mosquito-stage-structured malaria models and their global dynamics. SIAM J Appl Math 72(4):1213–1237
Arino J, Ducrot A, Zongo P (2012) A metapopulation model for malaria with transmission-blocking partial immunity in hosts. J Math Biol 64:423–448
Bacaër N, Ait Dads EH (2012) On the biological interpretation of a definition for the parameter \(R_0\) in periodic population models. J Math Biol 65:601–621
Bacaër N, Guernaoui S (2006) The epidemic threshold of vector-borne diseases with seasonality. J Math Biol 53:421–436
Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjørnstad ON (2017) The importance of temperature fluctuations in understanding mosquito population dynamics and malaria risk. R Soc Open Sci 4:160969
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalryple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briët O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CLJ, Smith DL, Hay SI, Cibulskis RE, Gething PW (2006) The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 53:421–436
Bliman P-A, Soledad Aronna M, Coelho FC, da Silva MAHB (2018) Ensuring successful introduction of Wolbachia in natural populations of Aedes aegypti by means of feedback control. J Math Biol 76(5):1269–1300
Centers for Disease Control and Prevention. https://www.cdc.gov/malaria/about/biology/index.html
Chitnis N, Hyman JM, Cushing JM (2008) Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull Math Biol 70:1272–1296
Craig M, Snow R, le Sueur D (1999) A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol Today 15(3):105–111
Diekmann O, Heesterbeek JAP, Metz JAJ (1990) On the definition and the computation of the basic reproduction ratio \(R_0\) in the models for infectious disease in heterogeneous populations. J Math Biol 28:365–382
Hale JK, Verduyn Lunel SM (1993) Introduction to functional differential equations. Springer, New York
Heffernan JM, Lou Y, Steben M, Wu J (2014) Cost-effectiveness evaluation of gender-based vaccination programs against sexually transmitted infections. Discrete Contin Dyn Syst Ser B 19(2):447–466
Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45:371–391
Hirsch MW, Smith HL, Zhao X-Q (2001) Chain transitivity, attractivity, and strong repellors for semidynamical systems. J Dyn Differ Equ 13:107–131
Inaba H (2012) On a new perspective of the basic reproduction number in heterogeneous environments. J Math Biol 22:113–128
Koenraadt CJM, Takken W (2018) Integrated approach to malaria control. Science 359(6375):528–529
Koosha M, Vatandoost H, Karimian F, Choubdar N, Oshaghi MA (2018) Delivery of a genetically marked Serratia AS1 to medically important arthropods for use in RNAi and paratransgenic control strategies. Microb Ecol. https://doi.org/10.1007/s00248-018-1289-7
Liang X, Zhang L, Zhao X-Q (2007) Basic reproduction ratios for periodic abstract functional differential equations (with application to a spatial model for Lyme disease). J Dyn Differ Equ. https://doi.org/10.1007/s10884-017-9601-7
Lou Y, Zhao X-Q (2011) Modelling malaria control by introduction of larvivorous fish. Bull Math Biol 73:2384–2407
Macdonald G (1957) The epidemiology and control of malaria. Oxford University Press, London
Martens P, Niessen LW, Rotmans J, Jetten TH, McMichael AJ (1995) Potential impact of global climate change on malaria risk. Environ Health Perspect 103(5):458–464
Ndo C, Menze-Djantio B, Antonio-Nkondjio C (2011) Awareness, attitudes and prevention of malaria in the cities of Douala and Yaoundé (Cameroon). Parasites Vectors. https://doi.org/10.1186/1756-3305-4-181
Ngarakana-Gwasira ET, Bhunu CP, Mashonjowa E (2014) Assessing the impact of temperature on malaria transmission dynamics. Afr Mat 25:1095–1112
Ngonghala CN, Mohammed-Awel J, Zhao R, Prosper O (2016) Interplay between insecticide-treated bed-nets and mosquito demography: implications for malaria control. J Theor Biol 397:179–192
Paaijmans KP, Cator LJ, Thomas MB (2009) Temperature-dependent pre-bloodmeal period and temperature-driven asynchrony between parasite development and mosquito biting rate reduce malaria transmission intensity. PLoS ONE 8(1):e55777
Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB (2010) Influence of climate on malaria transmission depends on daily temperature variation. PNAS 107(34):15135–15139
Rogers DJ, Randolph SE (2000) The global spread of malaria in a future, warmer world. Science 289:1763–1766
Ross R (1911) The prevention of malaria, 2nd edn. Murray, London
Ruang-Areerate T, Kittayapong P (2006) Wolbachia transinfection in Aedes aegypti: a potential gene driver of dengue vectors. Proc Natl Acad Sci 103(34):12534–12539
Rubel F, Brugger K, Hantel M, Chvala-Mannsberger S, Bakonyi T, Weissenbo H, Nowotny N (2008) Explaining Usutu virus dynamics in Austria: model development and calibration. Prev Vet Med 85:166–186
Shapiro LLM, Whitehead SA, Thomas MB (2017) Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol 15(10):e2003489
Smith HL (1995) Monotone dynamical systems: an introduction to the theory of competitive and cooperative systems, mathematical surveys and monographs 41. American Mathematical Society, Providence
Thieme HR (2009) Spectral bound and reproduction number for infinite-dimensional population structure and time heterogeneity. SIAM J Appl Math 70:188–211
Trape JF (2001) The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg 64(1 Suppl):12–17
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
Walter W (1997) On strongly monotone flows. Annales Polonici Mathematici LXVI:269–274
Wang W, Zhao X-Q (2008) Threshold dynamics for compartmental epidemic models in periodic environments. J Dyn Differ Equ 20:699–717
Wang X, Zhao X-Q (2017) A malaria transmission model with temperature-dependent incubation period. Bull Math Biol 79:1155–1182
Wang X, Zhao X-Q (2018) A climate-based malaria model with the use of bed nets. J Math Biol 77:1–25
Wang S, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M (2012) Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc Natl Acad Sci 109(31):12734–12739
Wang S, Dos-Santos ALA, Huang W, Liu KC, Oshaghi MA, Wei G, Agre P, Jacobs-Lorena M (2017) Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 357:1399–1402
World Health Organization (2018) Malaria vaccine: WHO position paper, January 2016-recommendations. Vaccine 36:3576–3577
Xiao Y, Zou X (2013a) Can multiple malaria species co-persist? SIAM J Appl Math 73(1):351–373
Xiao Y, Zou X (2013b) On latencies in malaria infection and their impact on the disease dynamics. Math Biosci Eng 10:463–481
Xiao Y, Zou X (2014) Transmission dynamics for vector-borne diseases in a patchy environment. J Math Biol 69:113–146
Yakob L, Yan G (2009) Modeling the effects of integrating larval habitat source reduction and insecticide treated nets for malaria control. PLoS ONE 4(9):e6921. https://doi.org/10.1371/journal.pone.0006921
Zhao X-Q (2003) Dynamical systems in population biology. Springer, New York, pp 46–49
Zhao X-Q (2017) Basic reproduction ratios for periodic compartmental models with time delay. J Dyn Differ Equ 29:67–82
Acknowledgements
We are very thankful to two anonymous referees for their insightful comments and helpful suggestions that greatly improved our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X., Zou, X. Modeling the Potential Role of Engineered Symbiotic Bacteria in Malaria Control. Bull Math Biol 81, 2569–2595 (2019). https://doi.org/10.1007/s11538-019-00619-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-019-00619-8