Abstract
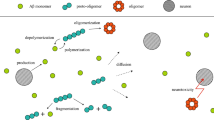
The aggregation of amyloid-𝛽 (A𝛽) proteins through their self-assembly into oligomers, fibrils, or senile plaques is advocated as a key process of Alzheimer’s disease. Recent studies have revealed that metal ions play an essential role in modulating the aggregation rate of amyloid-𝛽 (A𝛽) into senile plaques because of high binding affinity between A𝛽 proteins and metal ions. In this paper, we proposed a mathematical model as a set of coupled kinetic equations that models the self-assembly of amyloid-𝛽 (A𝛽) proteins in the presence of metal ions. The numerical simulations capture four timescales in the A𝛽 dynamics associated with three important events which include the formation of the amyloid–metal complex, the homogeneous aggregation of the amyloid–metal complexes, and the non-homogeneous aggregation of the amyloid–metal complexes. The method of singular perturbation is used to identify these timescales in the framework of slow–fast systems.


Similar content being viewed by others
References
ADS (2016) Alzheimers statistics. Alzheimers.net. http://www.alzheimers.net/resources/alzheimers-statistics/
Bica L, Crouch PJ, Cappai R, White AR (2009) Metallo-complex activation of neuroprotective signalling pathways as a therapeutic treatment for Alzheimer’s disease. Mol Biosyst 5:134–142
Bolognin S, Drago D, Messori L, Zatta P (2009) Chelation therapy for neurodegenerative diseases. Med Res Rev 29(4):547–570
Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75(1):333–366
Cohen SIA, Vendruscolo M, Dobson CM, Knowles TPJ (2011a) Nucleated polymerization with secondary pathways. II. Determination of self-consistent solutions to growth processes described by non-linear master equations. J Chem Phys 135(6):08B611
Cohen SIA, Vendruscolo M, Welland ME, Dobson CM, Terentjev EM, Knowles TPJ (2011b) Nucleated polymerization with secondary pathways. I. Time evolution of the principal moments. J Chem Phys 135(6):08B615
Cohen SI, Vendruscolo M, Dobson CM, Knowles TP (2012) From macroscopic measurements to microscopic mechanisms of protein aggregation. J Mol Biol 421(23):160–171 (Amyloid structure, function, and molecular mechanisms (part I))
Cohen SIA, Vendruscolo M, Dobson CM, Knowles TPJ (2013) The kinetics and mechanisms of amyloid formation. Wiley, Weinheim, pp 183–209
Crimins JL, Pooler A, Polydoro M, Luebke JI, Spires-Jones TL (2013) The intersection of amyloid beta and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer’s disease. Age Res Rev 12(3):757–763 (Synaptic Global positioning systems)
Cristóvão JS, Santos R, Gomes CM (2016) Metals and neuronal metal binding proteins implicated in Alzheimer’s disease. Oxid Med Cell Longev 2016:9812178
Faller P, Hureau C, Berthoumieu O (2013) Role of metal ions in the self-assembly of the alzheimers amyloid-\(\upbeta \) peptide. Inorg Chem 52(21):12193–12206
Faller P, Hureau C, La Penna G (2014) Metal ions and intrinsically disordered proteins and peptides: from cu/zn amyloid-\(\upbeta \) to general principles. Acc Chem Res 47(8):2252–2259
Hamley IW (2012) The amyloid beta peptide: a chemists perspective. role in alzheimers and fibrillization. Chem Rev 112(10):5147–5192
Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the united states (2010–2050) estimated using the 2010 census. Neurology 80(19):1778–1783
Hegde ML, Bharathi P, Suram A, Venugopal C, Jagannathan R, Poddar P, Srinivas P, Sambamurti K, Rao KJ, Scancar J, Messori L, Zecca L, Zatta P (2009) Challenges associated with metal chelation therapy in Alzheimer’s disease. J Alzheimers Dis 17(3):457–468
Jakob-Roetne R, Jacobsen H (2009) Alzheimer’s disease: from pathology to therapeutic approaches. Angew Chem Int Ed 48(17):3030–3059
Jarrett JT, Lansbury PTJ (1993) Seeding “one-dimensional crystallization”participating only in the process of nucleation. They are of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie. Cell 73(6):1055–1058
Karran E, Mercken M, Strooper BD (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 10(9):698–712
Kepp KP (2012) Bioinorganic chemistry of Alzheimers disease. Chem Rev 112(10):5193–5239
Knowles TPJ, Waudby CA, Devlin GL, Cohen SIA, Aguzzi A, Vendruscolo M, Terentjev EM, Welland ME, Dobson CM (2009) An analytical solution to the kinetics of breakable filament assembly. Science 326(5959):1533–1537
Leal SS, Botelho HM, Gomes CM (2012) Metal ions as modulators of protein conformation and misfolding in neurodegeneration. Coord Chem Rev 256:2253–2270
Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR (1998) Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 158(1):47–52
Mattson MP (2004) Pathways towards and away from Alzheimer’s disease. Nature 430(7000):631–639
Miller LM, Wang Q, Telivala TP, Smith RJ, Lanzirotti A, Miklossy J (2006) Synchrotron-based infrared and X-ray imaging shows focalized accumulation of cu and zn co-localized with \(\upbeta \)-amyloid deposits in Alzheimers disease. J Struct Biol 155(1):30–37
Mucke L, Selkoe DJ (2012) Neurotoxicity of amyloid \(\upbeta \)-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med 2(7):a006338
Oosawa F, Asakura S (1975) Thermodynamics of the polymerization of protein. Academic Press, London
Oosawa F, Kasai M (1962) A theory of linear and helical aggregations of macromolecules. J Mol Biol 4(1):10–21
Oosawa F, Asakura S, Hotta K, Imai N, Ooi T (1959) G-F transformation of actin as a fibrous condensation. J Polym Sci 37(132):323–336
Pithadia AS, Lim MH (2012) Metal-associated amyloid-\(\upbeta \) species in Alzheimer’s disease. Curr Opin Chem Biol 16:67–73 (Bioinorganic chemistry biocatalysis and biotransformation omics)
Prince M, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M (2016) World alzheimer report 2016: improving healthcare for people living with dementia. Technical report. https://www.alz.co.uk/research/world-report-2016
Rauk A (2009) The chemistry of Alzheimer’s disease. Chem Soc Rev 38:2698–2715
Sarell C (2010) The copper-amyloid-beta-peptide complex of Alzheimers disease: affinity, structure, fibril formation and toxicity. Ph.D. thesis, School of Biological and Chemical Sciences, University of London, Queen Mary, England
Turányi T, Tomlin AS (2014) Analysis of kinetic reaction mechanisms. Springer
Viles J H (2012) Metal ions and amyloid fiber formation in neurodegenerative diseases. Copper, zinc and iron in Alzheimer’s, Parkinson’s and Prion diseases. Coord Chem Rev 256(1920):2271–2284 (Metal Ions in neurodegenerative diseases)
Wilson MR, Yerbury JJ, Poon S (2008) Potential roles of abundant extracellular chaperones in the control of amyloid formation and toxicity. Mol BioSyst 4:42–52
Acknowledgements
The authors would like to thank Henry Research Foundation in Mississippi State University for the partial support of this project. The authors would like to thank Dr. Joe Hickey, MD, and Hickey Wellness Center for showing us the importance of this subject and inspiring the second author to start this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Proof of Theorem 2.1.
-
Evaluation of \({P_h(t)}\)
Setting \(i=j\) in Eq. (2), applying the summation over i and using the definition of \(P_h\) yields,
Since, \(\sum \limits _{i=3}^\infty f(t,i-1,i-1)=\sum \limits _{i=2}^\infty f(t,i,i)\), it is simplified to:
-
Evaluation of P(t)
Observe that,
and since,
it implies that,
-
Evaluation of \(M_h(t)\)
Since,
we get:
-
Evaluation of \(M_1(t)\)
Observe that,
and since,
we get:
-
Evaluation of \(M_2(t)\)
Observe that,
and since,
we get,
Rights and permissions
About this article
Cite this article
Asili, E., Yarahmadian, S., Khani, H. et al. A Mathematical Model for Amyloid-𝜷 Aggregation in the Presence of Metal Ions: A Timescale Analysis for the Progress of Alzheimer Disease. Bull Math Biol 81, 1943–1964 (2019). https://doi.org/10.1007/s11538-019-00583-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-019-00583-3