Abstract
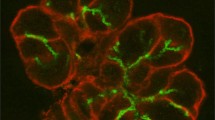
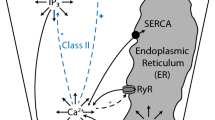
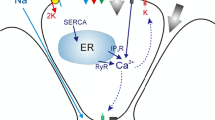
Salivary gland acinar cells use the calcium (\({\mathrm{Ca}}^{2+}\)) ion as a signalling messenger to regulate a diverse range of intracellular processes, including the secretion of primary saliva. Although the underlying mechanisms responsible for saliva secretion are reasonably well understood, the precise role played by spatially heterogeneous intracellular \({\mathrm{Ca}}^{2+}\) signalling in these cells remains uncertain. In this study, we use a mathematical model, based on new and unpublished experimental data from parotid acinar cells (measured in excised lobules of mouse parotid gland), to investigate how the structure of the cell and the spatio-temporal properties of \({\mathrm{Ca}}^{2+}\) signalling influence the production of primary saliva. We combine a new \({\mathrm{Ca}}^{2+}\) signalling model [described in detail in a companion paper: Pages et al. in Bull Math Biol 2018, submitted] with an existing secretion model (Vera-Sigüenza et al. in Bull Math Biol 80:255–282, 2018. https://doi.org/10.1007/s11538-017-0370-6) and solve the resultant model in an anatomically accurate three-dimensional cell. Our study yields three principal results. Firstly, we show that spatial heterogeneities of \({\mathrm{Ca}}^{2+}\) concentration in either the apical or basal regions of the cell have no significant effect on the rate of primary saliva secretion. Secondly, in agreement with previous work (Palk et al., in J Theor Biol 305:45–53, 2012. https://doi.org/10.1016/j.jtbi.2012.04.009) we show that the frequency of \({\mathrm{Ca}}^{2+}\) oscillation has no significant effect on the rate of primary saliva secretion, which is determined almost entirely by the mean (over time) of the apical and basal \([{\mathrm{Ca}}^{2+}]\). Thirdly, it is possible to model the rate of primary saliva secretion as a quasi-steady-state function of the cytosolic \([{\mathrm{Ca}}^{2+}]\) averaged over the entire cell when modelling the flow rate is the only interest, thus ignoring all the dynamic complexity not only of the fluid secretion mechanism but also of the intracellular heterogeneity of \([{\mathrm{Ca}}^{2+}]_i\). Taken together, our results demonstrate that an accurate multiscale model of primary saliva secretion from a single acinar cell can be constructed by ignoring the vast majority of the spatial and temporal complexity of the underlying mechanisms.













Similar content being viewed by others
References
Almassy J, Won JH, Begenisich TB, Yule DI (2012) Apical Ca\(^{2+}\)-activated potassium channels in mouse parotid acinar cells. J Gen Physiol 139(2):121–133. https://doi.org/10.1085/jgp.201110718
Almássy J, Siguenza E, Skaliczki M, Matesz K, Sneyd J, Yule DI, Nánási PP (2018) New saliva secretion model based on the expression of Na\(^+\)-K\(^+\) pump and K\(^+\) channels in the apical membrane of parotid acinar cells. Pflugers Arch 470(4):613–621. https://doi.org/10.1007/s00424-018-2109-0
Evans RL, Park K, Turner RJ, Watson GE, Nguyen H-V, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE (2000) Severe impairment of salivation in Na\(^{+}\)/K\(^{+}\)/2Cl\(^{-}\) cotransporter (NKCC1)-deficient mice. J Biol Chem 275(35):26,720–26,726. https://doi.org/10.1074/jbc.M003753200
Foskett J (1990) [Ca\(^{2+}\)]\(_i\) modulation of Cl\(^-\) content controls cell volume in single salivary acinar cells during fluid secretion. Am J Physiol Cell Physiol 259(6 Pt 1):C998–C1004. https://doi.org/10.1152/ajpcell.1990.259.6.C998
Foskett JK, Melvin JE (1989) Activation of salivary secretion: coupling of cell volume and [Ca\(^{2+}\)]\(_i\) in single cells. Science 244(4912):1582–1585
Foskett J, Roifman C, Wong D (1991) Activation of calcium oscillations by thapsigargin in parotid acinar cells. J Biol Chem 266(5):2778–2782
Gin E, Crampin EJ, Brown DA, Shuttleworth TJ, Yule DI, Sneyd J (2007) A mathematical model of fluid secretion from a parotid acinar cell. J Theor Biol 248(1):64–80. https://doi.org/10.1016/j.jtbi.2007.04.021
Giovannucci DR, Bruce JI, Straub SV, Arreola J, Sneyd J, Shuttleworth TJ, Yule DI (2002) Cytosolic Ca\(^{2+}\) and Ca\(^{2+}\)-activated Cl\(^-\) current dynamics: insights from two functionally distinct mouse exocrine cells. J Physiol 540(2):469–484
Mangos J, McSherry NR, Nousia-Arvanitakis S, Irwin K (1973) Secretion and transductal fluxes of ions in exocrine glands of the mouse. Am J Physiol 225(1):18–24. https://doi.org/10.1152/ajplegacy.1973.225.1.18
Martin C, Young J (1971) Electrolyte concentrations in primary and final saliva of the rat sublingual gland studied by micropuncture and catheterization techniques. Pflugers Archiv 324(4):344–360
Melvin JE, Yule D, Shuttleworth T, Begenisich T (2005) Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67:445–469. https://doi.org/10.1146/annurev.physiol.67.041703.084745
Nauntofte B (1992) Regulation of electrolyte and fluid secretion in salivary acinar cells. Am J Physiol 263(6):G823–G837. https://doi.org/10.1152/ajpgi.1992.263.6.G823
Pages N, Vera-Sigüenza E, Rugis J, Kirk V, Yule I D, Sneyd J (2018) A model of Ca\(^{2+}\) dynamics in an accurate reconstruction of parotid acinar cells. Bull Math Biol (submitted)
Palk L, Sneyd J, Shuttleworth TJ, Yule DI, Crampin EJ (2010) A dynamic model of saliva secretion. J Theor Biol 266(4):625–640. https://doi.org/10.1016/j.jtbi.2010.06.027
Palk L, Sneyd J, Patterson K, Shuttleworth TJ, Yule DI, Maclaren O, Crampin EJ (2012) Modelling the effects of calcium waves and oscillations on saliva secretion. J Theor Biol 305:45–53. https://doi.org/10.1016/j.jtbi.2012.04.009
Peña-Münzenmayer G, Catalán MA, Kondo Y, Jaramillo Y, Liu F, Shull GE, Melvin JE (2015) Ae4 (Slc4a9) anion exchanger drives Cl\(^-\)uptake-dependent fluid secretion by mouse submandibular gland acinar cells. J Biol Chem 290(17):10,677–10,688. https://doi.org/10.1074/jbc.M114.612895
Poulsen J, Bledsoe S (1978) Salivary gland K\(^+\) transport: in vivo studies with K\(^+\)-specific microelectrodes. Am J Physiol 234(1):E79–E83. https://doi.org/10.1152/ajpendo.1978.234.1.E79
Romanenko VG, Catalán MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE (2010) Tmem16A encodes the Ca\(^{2+}\)-activated Cl\(^-\) channel in mouse submandibular salivary gland acinar cells. J Biol Chem 285(17):12,990–13,001. https://doi.org/10.1074/jbc.M109.068544
Silva P, Stoff J, Field M, Fine L, Forrest J, Epstein F (1977) Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol 233(4):F298–F306. https://doi.org/10.1038/ki.1996.224
Soltoff S, McMillian M, Cantley L, Cragoe E, Talamo B (1989) Effects of muscarinic, alpha-adrenergic, and substance P agonists and ionomycin on ion transport mechanisms in the rat parotid acinar cell. the dependence of ion transport on intracellular calcium. J Gen Physiol 93(2):285–319. https://doi.org/10.1085/jgp.93.2.285
Swietach P, Zaniboni M, Stewart AK, Rossini A, Spitzer KW, Vaughan-Jones RD (2003) Modelling intracellular H\(^+\) ion diffusion. Prog Biophys Mol Biol 83(2):69–100
Tanimura A (2009) Mechanism of calcium waves and oscillations in non-excitable cells. Int J Dent Oral-Med Sci 8(1):1–11. https://doi.org/10.5466/ijoms.8.1
Vera-Sigüenza E, Catalàn MA, Peña-Münzenmayer G, E. Melvin J, Sneyd J (2018) A mathematical model supports a key role for ae4 (slc4a9) in salivary gland secretion. Bull Math Biol 80(2):255–282. https://doi.org/10.1007/s11538-017-0370-6
Young JA (1968) Microperfusion investigation of chloride fluxes across the epithelium of the main excretory duct of the rat submaxillary gland. Pflugers Archiv 303(4):366–374
Acknowledgements
This work was supported by the U.S. National Institutes of Health (NIDCR) Grant RO1DE019245-10 (ES, DY, and JS) and the Marsden Fund of the Royal Society of New Zealand Grant 3708441 (NP and JS). High-performance computing facilities and support were provided by the New Zealand eScience Infrastructure (NeSI). Funded jointly by NeSI’s collaborator institutions and through the Ministry of Business, Innovation and Employment’s Research Infrastructure programme (ES, NP, JS, and JR). Finally, we would like to thank NVIDIA Corporation for a K40 GPU grant (JR).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (mp4 121237 KB)
Appendices
Appendix
Fluxes of the Model
Water Fluxes
Where,
The parameter \(x_i\) denotes the amount of negatively charged ions with valence \(z = -1\)impermeable to the cellular PM. Its value is determined by imposing electroneutrality in the cellular compartment. Note that all compartments of the model are assumed electroneutral at all times. The parameter \(\varPsi _l\) in Eq. (26) represents the concentration of uncharged impermeable species present in the lumen, including (but not limited to) proteins such as amylase and big molecules like CO\(_2\).
Parameters of the Model
See the Table 1.
Rights and permissions
About this article
Cite this article
Vera-Sigüenza, E., Pages, N., Rugis, J. et al. A Mathematical Model of Fluid Transport in an Accurate Reconstruction of Parotid Acinar Cells. Bull Math Biol 81, 699–721 (2019). https://doi.org/10.1007/s11538-018-0534-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-0534-z