Abstract
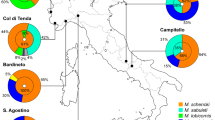
A model of interspecific host competition in a system with one parasite (butterfly—Maculinea) and multiple potential hosts (ants—Myrmica) is presented. Results indicate that host interspecific competition increases the occurrence of multiple host behaviour in Maculinea natural populations but decreases the ability of the parasite populations to adapt to the most abundant host species. These qualitative predictions were compared with data on host specificity, with good agreement. Analysis of the data also indicates that Maculinea teleius and Maculinea arion respond differently to changes in relative host abundances. Maculinea teleius shows a larger fraction of sites where it displays multiple host behaviour and a larger fraction of sites where the niches of the hosts overlap. In some instances, Maculinea teleius is adapted to Myrmica hosts that are present in lower frequencies. Maculinea arion is locally more host-specific and occurs at sites where host interspecific competition is unlikely and is more frequently adapted to the most abundant host species.






Similar content being viewed by others
References
Akino T, Knapp JJ, Thomas JA, Elmes GW (1999) Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc R Soc Lond B 266:1419–1426
Assis RA, Bonelli S, Witek M, Barbero F, Casacci LP, Baletto E, Venturino E, Ferreira WC Jr (2012) A model for the evolution of parasite-host interactions based on the maculinea-myrmica system: numerical simulations and multiple host behavior. Nonlinear Anal Real World Appl 13:1507–1524
Augusiaka J, Van den Brink PJ, Grimm V (2014) Merging validation and evaluation of ecological models to “evaludation”: a review of terminology and a practical approach. Ecol Model 280:117–128
Barbero F, Casacci LP (2015) Butterflies that trick ants with sounds. Phys Today 68:64
Barbero F, Thomas JA, Bonelli S, Balletto E, Schönrogge K (2009) Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 323:782–785
Bonelli S, Crocetta A, Barbero F, Balletto E (2005) Oviposition behaviour in the myrmecophilous butterfly Maculinea alcon (Lepidoptera: Lycaenidae). In: Settele J, Kühn E, Thomas JA (eds) Species ecology along a European gradient: maculinea butterflies as a model. Studies on the ecology and conservation of butterflies in Europe, vol 2. Pensoft Publishers, Sofia, pp 65–68
Casacci LP, Witek M, Barbero F, Patricelli D, Solazzo G, Balletto E, Bonelli S (2011) Habitat preferences of Maculinea arion and its Myrmica host ants: implications for habitat management in Italian Alps. J Insect Conserv 15:103–110
Clarke RT, Thomas JA, Elmes GW, Hochberg ME (1997) The effects of spatial patterns in habitat quality on community dynamics within a site. Proc R Soc Lond B Biol Sci 264:347–354
Clarke RT, Thomas JA, Elmes GW, Wardlaw JC, Munguira ML, Hochberg ME (1998) Population modelling of the spatial interactions between Maculinea rebeli, their initial foodplant Gentiana cruciata and Myrmica ants within a site. J Insect Conserv 2:29–37
Cole BJ (2009) The ecological setting of social evolution: the demography of ant populations. In: Gadau J, Fewell J (eds) Organization of insect societies. Harvard University Press, Cambridge, pp 74–104
Czechowski W, Radchenko A, Czechowska W, Vepsäläinen K (2012) The ants of Poland with reference to the myrmecofauna of Europe, vol 4. Natura Optima Dux Foundation, Warszawa
Elmes GW (1973) Observations on the density of queens in natural colonies of Myrmica rubra L. (Hymenoptera: Formicidae). J Anim Ecol 42:761–771
Elmes GW (1974) The effect of colony population on caste size in three species of Myrmica (Hymenoptera: Formicidae). Insectes Soc 21:213–230
Elmes GW, Wardlaw JC (1982) A population study of the ants Myrmica sabuleti and Myrmica scabrinodis, living at two sites in the south of england: I. A comparison of colony populations. J Anim Ecol 51(2):651–664
Elmes GW, Thomas JA, Wardlaw JC (1991a) Larvae of Maculinea rebeli, a large blue buttery, and their Myrmica host ants: wild adoption and behaviour in ant-nests. J Zool 223:447–460
Elmes GW, Wardlaw JC, Thomas JA (1991b) Larvae of Maculinea rebeli, a large blue buttery, and their Myrmica host ants: patterns of caterpillar growth and survival. J Zool 224:79–92
Elmes GW, Hammarstedt O, Martin J, Thomas JA, Van Der Made JG (1994) Differences in host-ant specificity between spanish, dutch and swedish populations of the endangered butterfly Maculinea alcon (DENIS at SCHIFF.)(Lepidoptera). Memorab Zool 48:55–68
Elmes GW, Clarke RT, Thomas JA, Hochberg ME (1996) Empirical tests of specific predictions made from a spatial model of the population dynamics of Maculinea rebeli, a parasitic buttery of red ant colonies. Acta Oecol 17:61–80
Elmes GW, Thomas JA, Wardlaw JC, Hochberg ME, Clarke RT, Simcox DJ (1998) The ecology of Myrmica ants in relation to the conservation of Maculinea butterflies. J Insect Conserv 2:67–78
Elmes GW, Thomas JA, Clarke R, Knapp J (2002) Interspecific differences in cuticular hydrocarbon profiles of Myrmica ants are sufficiently consistent to explain host specificity by Maculinea (large blue) butterflies. Oecologia 130:525–535
Fayle TM, Eggleton P, Manica A, Yusah KM, Foster WA (2015) Experimentally testing and assessing the predictive power of species assembly rules for tropical canopy ants. Ecol Lett 18:254–262
Gallé L (1986) Habitat and niche analysis of grassland ants (Hymenoptera: Formicidae). Entomol Generalis 11:197–211
Griebeler EM, Seitz A (2002) An individual based model for the conservation of the endangered large blue butterfly, Maculinea arion (Lepidoptera: Lycaenidae). Ecol Model 156:43–60
Hayes MP (2015) The biology and ecology of the large blue butterfly phengaris (Maculinea) arion: a review. J Insect Conserv 19:1037–1051
Hochberg ME, Thomas JA, Elmes GW (1992) A modelling study of the population dynamics of a large blue butterfly, Maculinea rebeli, a parasite of red ant nests. J Anim Ecol 61:397–409
Hochberg ME, Clarke RT, Elmes GW, Thomas JA (1994) Population dynamic consequences of direct and indirect interactions involving a large blue butterfly and its plant and red ant hosts. J Anim Ecol 63:375–391
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Kudrna O, Harpke A, Lux K, Pennerstorfer J, Schweiger O, Settele J, Wiemers M (2011) The distribution atlas of butterflies in Europe. Gesellschaft für Schmetterlinsschulz, Halle
Nash DR, Als TD, Maile R, Jones GR, Boomsma JJ (2008) A mosaic of chemical coevolution in a large blue butterfly. Science 319:88–90
Nowicki P, Settele J, Thomas JA, Woyciechowski M (2005) A review of population structure of maculinea butterflies. In: Settele J, Kühn E, Thomas JA (eds) Studies in the ecology and conservation of butterflies in Europe, species ecology along a European gradient: Maculinea butterflies as a model, vol 2. Pensoft Publishers, Sofia, pp 144–149
Nowicki P, Pepkowska A, Kudlek J, Skórka P, Witek M, Settele J, Woyciechowski M (2007) From metapopulation theory to conservation recommendations: lessons from spatial occurrence and abundance. Biol Conserv 140:119–129
Nowicki P, Bonelli S, Barbero F, Balletto E (2009) Relative importance of density-dependent regulation and environmental stochasticity for butterfly population dynamics. Oecologia 161:227–239
Patricelli D, Barbero F, La Morgia V, Casacci LP, Witek M, Balletto E, Bonelli S (2011) To lay or not to lay: oviposition of Maculinea arion in relation to Myrmica ant presence and host plant phenology. Anim Behav 82:791–799
Patricelli D, Barbero F, Occhipinti A, Bertea CM, Bonelli S, Casacci LP, Zebelo SA, Crocoll CM, Gershenzon J, Maffei ME, Thomas JA, Balletto E (2015) Plant defences against ants provide a pathway to social parasitism in butterflies. Proc R Soc Lond B 282:20151111
Pech P, Fric Z, Konvicka M (2007) Species-specificity of the Phengaris (Maculinea)-Myrmica host system: fact or myth? (Lepidoptera: Lycaenidae; Hymenoptera: Formicidae). Sociobiology 50:83–1003
Radchenko A, Elmes GW (2010) Myrmica ants of the old world. Natura Optima Dux Foundation, Warsaw
Read TR, Cressie NA (1988) Goodness of fit statistics for discrete multivariate data. Springer, New York
Sala M, Casacci LP, Balletto E, Bonelli S, Barbero F (2014) Variation in butterfly larval acoustics as a strategy to infiltrate and exploit host ant colony resources. PLoS ONE 9:e94341
Savolainen R, Vepsäläinen K (1988) A competition hierarchy among boreal ants: impact on resource partitioning and community structure. Oikos 51:135–155
Schlick-Steiner BC, Steiner FM, Höttinger H, Nikiforov A, Mistrik R, Schafellner C, Baier P, Christian E (2004) A butterfly’s chemical key to various ant forts: intersection-odour or aggregate-odour multi-host mimicry? Naturwissenschaften 91:209–214
Schönrogge K, Wardlaw JC, Peters AJ, Everett S, Elmes GW (2004) Changes in chemical signature and host specificity from larval retrieval to full social integration in the myrmecophilous butterfly Maculinea rebeli. J Chem Ecol 30:91–107
Sielezniew M, Stankiewicz A (2008) Myrmica sabuleti (Hymenoptera: Formicidae) not necessary for the survival of the population of Phengaris (Maculinea) arion (Lepidoptera: Lycaenidae) in eastern poland: lower host-ant specificity or evidence for geographical variation of an endangered social parasite? Eur J Entomol 105:637–641
Sielezniew M, Dziekańska I, Stankiewicz-Fiedurek AM (2010a) Multiple host-ant use by the predatory social parasite Phengaris (=Maculinea) arion (Lepidoptera, Lycaenidae). J Insect Conserv 14:141–149
Sielezniew M, Patricelli D, Dziekańnska I, Barbero F, Bonelli S, Casacci LP, Witek M, Balletto E (2010b) The first record of Myrmica lonae (hymenoptera: Formicidae) as a host of socially parasitic large blue butterfly Phengaris (Maculinea) arion (Lepidoptera: Lycaenidae). Sociobiology 56:465–475
Sielezniew M, Wlostowski M, Dziekańska I (2010c) Myrmica schencki (hymenoptera: Formicidae) as the primary host of Phengaris (Maculinea) arion (Lepidoptera: Lycaenidae) at heathlands in Eastern Poland. Sociobiology 55:95–106
Stankiewicz A, Sielezniew M (2002) Host specificity of Maculinea teleius Bgstr. and M. nausithous Bgstr. (Lepidoptera: Lycaenidae): the new insight. Ann Zool 52:403–408
Tartally A (2008) Myrmecophily of Maculinea butterflies in the carpathian basin (Lepidoptera: Lycaenidae). Ph.D. thesis, University of Debrecen, Debrecen
Tartally A, Csösz S (2004) Adatok a magyarországi Maculinea fajok (Lepidoptera: Lycaenidae) hangyagazdáiról (data on the ant hosts of the maculinea butterflies (Lepidoptera: Lycaenidae) of hungary). Természetvédelmi Közlemények 11:309–317
Tartally A, Varga Z (2008) Host ant use of Maculinea teleius in the carpathian-basin (Lepidoptera: Lycaenidae). Acta Zool Acad Sci Hung 54:257–268
Tartally A, Nash DR, Lengyel S, Varga Z (2008) Patterns of host ant use by sympatric populations of Maculinea alcon and M. “rebeli” in the carpathian basin. Insectes Soc 55:370–381
Thomas JA, Elmes GW, Schönrogge K, Simcox DJ, Settele J (2005a) Primary hosts, secondary hosts and “non host”: common confusion in the interpretation of host specificity in Maculinea butterflies and other social parasites of ants. In: Studies on the ecology and conservation of butterflies in Europe, vol 2. Species ecology along a European gradient: Maculinea butterflies as a model. Pensoft Publishers, Sofia, pp 99–104
Thomas JA, Schönrogge K, Elmes GW, Fellowes MD, Holloway GJ, Rolff J (2005b) Specializations and host associations of social parasites of ants. In: Proceedings of the royal entomological society’s 22nd symposium of insect evolutionary ecology. CABI Publishing, Reading, pp 479–518
Thomas JA (1980) Why did the large blue become extinct in Britain? Oryx 15:243–247
Thomas JA, Elmes GW (1998) Higher productivity at the cost of increased host-specificity when Maculinea butterfly larvae exploit ant colonies through trophallaxis rather than by predation. Ecol Entomol 23:457–464
Thomas JA, Settele J (2004) Evolutionary biology: butterfly mimics of ants. Nature 432:283–284
Thomas JA, Wardlaw JC (1992) The capacity of a Myrmica ant nest to support a predacious species of Maculinea rebeli inhabitants in ant nests. Oecologia 91:101–109
Thomas JA, Elmes GW, Wardlaw JC, Woyciechowski M (1989) Host specificity among Maculinea butterflies in Myrmica ant nests. Oecologia 79:452–457
Thomas JA, Munguira ML, Martin J, Elmes GW (1991) Basal hatching in Maculinea butterflies eggs: a consequence of advanced myrmecophily? Biol J Linn Soc 44:175–184
Thomas JA, Elmes GW, Clarke RT, Kim KG, Munguira ML, Hochberg ME (1997) Field evidence and model predictions of butterfly-mediated apparent competition between gentian plants and red ants. Acta Oecol 18:671–684
Thomas JA, Simcox DJ, Clarke RT (2009) Successful conservation of a threatened Maculinea butterfly. Science 325:80–83
Thomas JA, Elmes GW, Sielezniew M, Stankiewicz-Fiedurek A, Simcox DJ, Steele J, Schönrogge K (2013) Mimetic host shifts in an endangered social parasite of ants. Proc R Soc Lond B 280:20122336
van Swaay CAM, Collins S, Dusej G, Maes D, Munguira ML, Rakosy L, Ryrholm N, Sasic M, Settele J, Thomas JA, Verovnik R, Verstrael T, Warren MS, Wiemers M, Wynhoff I (2012) Do’s and don’ts for butterflies of the habitats directive of the European union. Nat Conserv 1:73–153
Völkl R, Schiefer T, Bräu M, Stettmer C, Binzenhöfer B, Settele J (2008) Auswirkungen von mahdtermin und-turnus auf populationen der ameisen-bläulinge Maculinea nausithous und Maculinea teleius-ergebnisse mehrjähriger habitatanalysen in Bayern. Naturschutz Landsch 40:147–155
Waltman P (1983) Competition models in population biology. SIAM, Philadelphia
Wardlaw J, Elmes GW, Thomas JA (1998) Techniques for studying Maculinea butterflies: II. Identification guide to Myrmica ants found on Maculinea sites in Europe. J Insect Conserv 2:119–127
Witek M, Sliwinska EB, Skórka P, Nowicki P, Wantuch M, Vrabec V, Settele J, Woyciechowski M (2008) Host ant specificity of large blue butterflies phengaris (Maculinea) (Lepidoptera:Lycaenidae) inhabiting humid grasslands of East-central Europe. Eur J Entomol 105:871–877
Witek M, Nowicki P, Śliwinska BE, Skórka P, Settele J, Schönrogge K, Woyciechowski M (2010) Local host ant specificity of Phengaris (Maculinea teleius) butterfly, an obligatory social parasite of Myrmica ants. Ecol Entomol 35:370–381
Witek M, Barbero F, Markó B (2014) Myrmica ants host highly diverse parasitic communities: from social parasites to microbes. Insectes Soc 61:307–323
Woyciechowski M, Slowik J, Muehlenberg M (2006) Hosts of the butterfly Maculinea teleius among Myrmica ants in northern Mongolia (Lepidoptera: Lycaenidae; Hymenoptera: Formicidae). Sociobiology 48:493–502
Wynhoff I (1998) The recent distribution of the european Maculinea species. J Insect Conserv 2:15–27
Acknowledgements
Raul Abreu de Assis acknowledges the support of CNPQ, Grant No. 203654/2014-5, and the support of FAPEMAT, Edital Universal 005-2015, Process No. 204271/2015. The authors thank Magdalena Witek and two anonymous referees for suggestions and improvements on the previous versions of this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix A: Testing the Significance of the Patterns
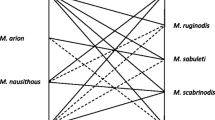
In Sect. 3.2 we defined two classes of interspecific competition, C and NC, which are based on the relations between ecological niches of 11 species. Each pair of species was classified to be either in a state of “higher chance of competition” or “smaller chance of competition”, using the information contained in the literature (Elmes et al. 1998; Radchenko and Elmes 2010) about the niche of each species. If there was an estimate of a significant niche overlap [see Fig. 1 in Elmes et al. (1998)], the species were said to be in class C, while if niche overlap was estimated as small or non-existent, the species we assigned to the disjoint class NC.
For each simulation, a random matrix \(\hbox {RM}_\mathrm{C}\) is generated and the total score of V’s (as in Table 3) is computed. The figure represents the frequencies of the obtained scores if the relations between the species were randomly attributed. Of the total 1000 simulations none achieved the score of 30/30, attained by the matrix \(M_\mathrm{C}\), indicating the low probability that the patterns observed are due to chance
The matrix \(M_\mathrm{C}\) (see Table 4) is used to separate the data from the sites into categories, which are then analysed for the patterns (observations 1–3, in Sect. 3) described by the theoretical model. Therefore, to test whether the patterns did not arise simply by chance, we simulated the results that would be obtained if the symmetric binary matrix \(M_\mathrm{C}\) was created using random associations between the Myrmica species (that is, that do not take into account the biological information on niches).
\(M_\mathrm{C}\) has 14 nonzero elements and the diagonal is zero. Since it is symmetric, to create a correspondent random matrix, it is enough to generate 7 entries equal to 1 above the diagonal. If these entries are placed at random, the matrix thus obtained represents a random association of Myrmica species, not based on ecological properties. The random matrix represents an arbitrary classification of the Myrmica species.
We simulated 1000 random associations, and for each, we repeated the analysis presented in Table 3, calculating the total number of agreements between theoretical predictions and empirical data. For these cases where some statistic or test could not be calculated (if the class had no members or not enough to calculate a correlation, for example), the test was counted as positive, that is the evaluation of the scores of the random matrices was biased in favour of the random attributions. In none of the 1000 simulations we obtained a score equal to the one obtained by the matrix \(M_\mathrm{C}\). In Fig. 7 we present a histogram with the distribution of results.
The results from these simulations strongly indicate that the agreement with all three theoretical observations has a very small probability of being obtained by chance, meaning that the patterns are robust.
Appendix B: Mathematical Analysis
Change of variables in Eq. (2): \(y_1=p_1/(p_1+p_2)\), \(y_2=p_2/(p_1+p_2)=1-y_1\). Results in a three-dimensional system:
We will exclude equilibria with both \(h_1=h_2=0\), but numerical simulations with a model that includes small perturbation in the denominator (\(\gamma _1h_1+\gamma _2 \sigma h_2+\epsilon \)) present results very similar to the original, indicating that the singularity is not a problem for modelling purposes. Also, if both populations were absent, the ecological problem loses its meaning, since in the absence of hosts the parasite cannot be present.
There are 6 equilibria for \(h_1+h_2>0\):
\(A=(0,h_{2A},0)\), with \(h_{2A}=1-\gamma _2\), feasible for
\(B=(0,h_{2B},y_{1B})\), with \(h_{2B}=1\), \(y_{1B}=1\), always feasible.
\(F=(h_{1F},0,0)\), with \(h_{1F}=1\), always feasible.
\(C=(h_{1C},0,y_{1C})\), with \(h_{1C}=1-\gamma _1\), \(y_{1C}=1\), feasible for
\(D=(h_{1D},h_{2D},0)\), with
feasible for one of the alternative sets of conditions
Finally we have the coexistence point
\(E=(h_{1E},h_{2E},y_{1E})\) that gives rise to two different cases.
\(E_a=(h_{1Ea},h_{2Ea},y_{1Ea})\) with \(y_{1Ea}=1\),
Alternatively, we can impose \(\gamma _1 h_1 = \gamma _2 \sigma h_2\) to satisfy the third equilibrium equation, and in such case we obtain
The Jacobian of (3) is
with
At A the eigenvalues of the Jacobian evaluated at this point J(A) are \(-1\), \(\lambda _1 (1-c h_{2A})\), \(-\lambda _2 h_{2A}\), entailing the stability conditions \(1<c h_{2A}\) and \(-\lambda _2 h_{2A}<0 \) or, extensively,
For the equilibrium B we find \(-\lambda _2\), \(-\lambda _1 (\gamma _1 +c-1)\) and 1, so its unconditional stability is obtained.
For the equilibrium F we find instead \(-\lambda _1\), \(\lambda _2 (1-c - \gamma _2)\) and 1, so the equilibrium is also unconditionally stable.
The point C again leads to an immediate evaluation of the eigenvalues: \(-\lambda _1 h_{1C}\), \(\lambda _2- c \lambda _2 h_{1C}\), \(-1\), giving a stable equilibrium for
which explicitly become
The Jacobian at the equilibrium D has the explicit eigenvalue
from which the first stability condition
The Routh–Hurwitz conditions on the remaining 2 by 2 minor are
which explicitly can be rewritten as
or
and
or
At coexistence \(E_a\), the Jacobian simplifies a bit; we find
while at \(E_b\) instead we have
For \(E_a\) we have one eigenvalue, and by Routh–Hurwitz conditions on the remaining minor, the one on the trace is satisfied
which explicitly can be rewritten as
or
while for the determinant we find
which is positive only if we require
or
At \(E_b\) the Jacobian is almost full, but the condition on the trace is the same as above (11). For the determinant we find
which is positive only if we require
Further, the condition on the sum of minors leads to the following inequality
For a Hopf bifurcation, condition (13) becomes an equality, for some chosen bifurcation parameter.
Appendix C: Parameter Estimation
The parameters to be estimated are: selection pressures (\(\gamma _i\)), relative rates of reproduction (\(\lambda _i\)), the relative abundance of hosts (\(\sigma \)) and maximum impact of interspecific competition (c).
The selection pressures, \(\gamma _i\), represent the maximum impact on fitness by the population of parasites on one host. For the Maculinea–Myrmica system it is limited by several factors, such as the percentage of area that the food plants occupy in the habitat of the host. This limited distribution of the food plants limits the fraction of hosts exposed to the parasite, given that the maximum foraging distance of ant colonies is around 2 m (Elmes et al. 1998). There is also a maximum parasite impact on the reproduction rate of the host, because most nests survive parasite exploitation and, in some cases, part of the ant brood is not available to be parasitized (Thomas and Wardlaw 1992). In Griebeler and Seitz (2002) the average of the area covered by the food plants (Thymus pulegioides) at different sites was less than 11.75% (minimum of 1%, maximum of 10%, SD 11.56%). Hochberg et al. (1994) used values between 50 and 70% for the percentage of nests exposed to the parasite, and Elmes and Wardlaw (1982) have shown that, at a site where Maculinea arion was present, colonies of Myrmica sabuleti had an average of 183 workers, while at a site where the parasite was absent the average was of 317 workers. For the same site, Thomas and Wardlaw (1992) found an average nest size of 180 (SD 164) workers. Given that the number of workers is closely correlated with the production of reproductive female individuals (Elmes 1973; Elmes and Wardlaw 1982), we can estimate that the average maximum impact on fitness for that particular site is below 43%. All the above information suggests that parasite pressure, in general, is below 0.7.
The relative reproduction rates \(\lambda _i=r_i/r_\mathrm{P}\) can be approximated using the estimates of \(R_0\), which in Elmes et al. (1996) denotes the maximum yearly reproduction rate of the parasite species, for the parasite and host species (in logistic growth, \(r_\mathrm{P}\approx R_0\)). In a modelling study, Hochberg et al. (1992) estimate \(R_0\) as 6.2 for Maculinea rebeli, while Thomas et al. (2009) have an estimate of 5.49 for Maculinea arion. For the Myrmica species, Elmes (1973) uses a value of 1.6 for Myrmica rubra, while Hochberg et al. (1994) in a model with various Myrmica species adopts an interval between 1.3 and 2 (depending on environmental factors) for \(R_0\). These estimates suggest a value of \(\lambda _i\) in the interval 0.21–0.36 (a more conservative interval is from 0.14 to 0.40, using \(r_{\mathrm{logistic}}\approx \ln (R_0)\)).
The relative abundance of hosts (\(\sigma =K_2/K_1\)) is the carrying capacity of the site in relation to host 2 divided by the carrying capacity in relation to host 1. The studies quoted in Sect. 2.2 are usually conducted within the range of the food plants, and studies where the distribution of Myrmica nests outside this range are rare. Therefore, one can only work with hypotheses on the selection pressures and use the estimates of \(K_i\gamma _i\), which is actually what is measured by looking for host nests within the area close to food plants, to estimate \(\sigma \). For instance, if selection pressures are approximately equal, it is enough to divide the frequency of nests of host 2 found inside the food plant area by the frequency of nests of host 1 (\(M_2/M_1\)) to estimate \(\sigma \).
The maximum effect of interspecific competition (c) is the maximum fraction of population size that may be reduced due to the presence of other host species. It depends on several factors: habitat distribution, behavioural variations among species and climatic variation (Hölldobler and Wilson 1990; Elmes et al. 1998; Radchenko and Elmes 2010). Hochberg et al. (1994) have worked with a habitat superposition of 50%, but the great variability of the relative abundance of different species (as illustrated in Sect. 2.2) suggests that the overlapping patterns may be more variable and unpredictable than the simple gradient pattern used in the models. In our simulations, we allow c to vary in the interval (0, 1), both to incorporate this variability and as a way to better explore the effects of interspecific competition.
Appendix D: Random Parameters and Initial Conditions
Given the range of parameters presented in Table 2, to investigate the influence of interspecific competition on host specificity, we chose 11 values of c in the set \(S=\{0,0.1,\dots ,1\}\) and, for each value of c, took random combinations of parameters and initial conditions according to the rules given below:
-
\(\gamma \): uniform distribution within its range: [0.01, 0.7].
-
\(\lambda \): uniform distribution within its range: [0.14, 0.4].
-
\(p_1(0)\), \(p_2(0)\), \(h_1(0)\) and \(h_2(0)\): uniform in [0, 1].
-
\(T_\mathrm{H}=K_1/(K_1+K_2)=1/(1+\sigma )\): uniform in [0, 1].
The reason for sampling \(T_\mathrm{H}\) instead of \(\sigma \) is to generate the various scenarios of different host abundance, without bias.
For each different value of c, 1000 trials were run, each with a new set of random parameters and initial conditions. Of the total 11000 simulations, 790 (\(\approx 7\%\)) failed numerically and were discarded. In Figs. 8 and 9 we present results of 100 simulations for each value of c in \(\{0,0.2,0.4,0.6,0.8,1\}\).
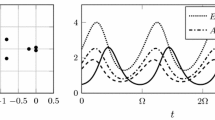
Relation between the theoretical host abundance \(T_\mathrm{H}=K_1/(K_1+K_2)=1/(1+\sigma )\) and the theoretical parasite distribution \(T_\mathrm{P}=P_1/(P_1+P_2)\). Simulations of model 2 for random \(\gamma \), \(\lambda \) and initial conditions \(h_1(0),h_2(0),p_1(0)\) and \(p_2(0)\). 100 points for each value of \(c \in \{0, 0.2, 0.4\}\)
Relation between the theoretical host abundance \(T_\mathrm{H}=K_1/(K_1+K_2)=1/(1+\sigma )\) and the theoretical parasite distribution \(T_\mathrm{P}=P_1/(P_1+P_2)\). Simulations of model 2 for random \(\gamma \), \(\lambda \) and initial conditions \(h_1(0),h_2(0),p_1(0)\) and \(p_2(0)\). 100 points for each value of \(c \in \{0.6, 0.8, 1\}\)
Figures 8 and 9 indicate that the observations made for the simulations performed with fixed parameters are also valid for the simulations with random parameters, that is, even with conditions changing from site to site, the patterns observed should still hold. To test more precisely observation 1 we calculated the regression slope for \(T_\mathrm{H}\) and \(T_\mathrm{P}\) in the simulations for each value of \(c \in S\). Results are presented in Fig. 10.
To support observation 2, we calculated the ratio of points in the region \((0,0.5)\times [0.5,1]\cup (0.5,1)\times [0,0.5]\) over the total points for each value of c. It represents the simulations where the primary host was less abundant than the secondary. The simulations with \(c=1\) showed approximately 50% of such occurrence, with \(c=0.9\) around 3%, and for the other values of c, percentages were below 0.2%.
Now we can compare the observations gathered from the simulation results with the data in Sect. 2.2; in particular, we are interested in testing observations 1, 2 and 3 of this section.
Appendix E: Model Validation with Field Data
The “validation” used here for the model is a complex process found in Augusiaka et al. (2014), in which a unifying terminology has been proposed. We focused primarily on model output validation of the qualitative predictions presented in Sect. 3.2. The parameters of the model were estimated from the literature, and no fine-tuning is necessary to observe the patterns predicted. Therefore, the dataset of 32 different sites can be considered independent of model calibration, making it adequate for validation.
In Table 4 we present the classification of the relations between the species as a \(11\times 11\) binary matrix, \(M_\mathrm{C}\) with 1 representing pertinence to class C and 0 non-pertinence. Since the classes are disjoint, one matrix is enough to describe both classes.
Using the classification, as in Table 4, we have two categories of interspecific competition, C, NC. For each category, if we assign a corresponding coefficient of interspecific competition \(c_\mathrm{c}\) and \(c_\mathrm{nc}\), the coefficients would obey the relation: \(c_\mathrm{c}>c_\mathrm{nc}\), as in Fig. 11. Here \(c_\mathrm{c}>c_\mathrm{nc}\) implies that interspecific competition in the class C is stronger than in class NC.
From the ordering of the coefficients, Fig. 10 suggests that the correlations between the theoretical primary host abundance (\(T_\mathrm{H}\)) and the theoretical parasite distribution (\(T_\mathrm{P}\)) should decrease as c increases. Given the classes C and NC and the expected relations between the coefficients \(c_\mathrm{c}\) and \(c_\mathrm{nc}\), we should expect the slope of the linear regression between the empirical variables, \(E_\mathrm{H}\) and \(E_\mathrm{P}\), to increase when comparing the class C with NC. Such are the results presented in Sect. 3.2.
Rights and permissions
About this article
Cite this article
de Assis, R.A., Casacci, L.P., Bonelli, S. et al. Effects of Host Interspecific Interaction in the Maculinea–Myrmica Parasite–Host System. Bull Math Biol 80, 2378–2407 (2018). https://doi.org/10.1007/s11538-018-0466-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-0466-7
Keywords
- Parasite
- Host
- Maculinea
- Myrmica
- Model
- Differential equations
- Ecology
- Multiple host behaviour
- Management
- Butterfly conservation