Abstract
We present an analysis of an avian flu model that yields insight into the roles of different transmission routes in the recurrence of avian influenza epidemics. Recent modelling work suggests that the outbreak periodicity of the disease is mainly determined by the environmental transmission rate. This conclusion, however, is based on a modelling study that only considers a weak between-host transmission rate. We develop an approximate model for stochastic avian flu epidemics, which allows us to determine the relative contribution of environmental and direct transmission routes to the periodicity and intensity of outbreaks over the full range of plausible parameter values for transmission. Our approximate model reveals that epidemic recurrence is chiefly governed by the product of a rotation and a slowly varying standard Ornstein–Uhlenbeck process (i.e. mean-reverting process). The intrinsic frequency of the damped deterministic version of the system predicts the dominant period of outbreaks. We show that the typical periodicity of major avian flu outbreaks can be explained in terms of either or both types of transmission and that the typical amplitude of epidemics is highly sensitive to the direct transmission rate.









Similar content being viewed by others
References
Alexander DJ (2000) A review of avian influenza in different bird species. Vet Microbiol. http://www.sciencedirect.com/science/article/pii/S0378113500001607
Alexander D J, Parsons G, Manvell R J (1986) Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathol J W.V.P.A 15(4):647–662
Allen EJ, Allen LJ, Arciniega A, Greenwood PE (2008) Construction of equivalent stochastic differential equation models. Stoch Anal Appl 26:274–297
Alonso D, McKane AJ, Pascual M (2007) Stochastic amplification in epidemics. J R Soc Interface 4(14):575–582
Aparicio JP, Solari HG (2001) Sustained oscillations in stochastic systems. Math Biosci 169(1):15–25
Baxendale PH, Greenwood PE (2011) Sustained oscillations for density dependent Markov processes. J Math Biol 63(3):433–457
Breban R, Drake JM (2009) The role of environmental transmission in recurrent avian influenza epidemics. PLoS Comp Biol 5(4):e1000346
Breban R, Drake JM, Stallknecht DE, Rohani P (2009) The role of environmental transmission in recurrent avian influenza epidemics Supporting Information. J Exp Biol 1–9
Clancy CF, O’Callaghan MJA, Kelly TC (2006) A multi-scale problem arising in a model of avian flu virus in a seabird colony. J Phys Conf Ser 55:45–54
Connor MB (1956) A historical survey of methods of solving cubic equations. http://scholarship.richmond.edu/masters-theses/114/
Danø S, Sø rensen P G, Hynne F (1999) Sustained oscillations in living cells. Nature 402(6759):320–322
Dykman MI, McClintock PVE (1998) What can stochastic resonance do? Nature 391(6665):344
Gang H, Ditzinger T, Ning CZ, Haken H (1993) Stochastic resonance without external periodic force. Phys Rev Lett 71(6):807–810
Garamszegi LZ, Møller AP (2007) Prevalence of avian influenza and host ecology. Proc R Soc B 274(1621):2003–2012
Gardiner CW (1986) Handbook of stochastic methods for physics, chemistry and the natural sciences. Applied Optics (vol. 25, p. 3145)
Greenwood PE, Gordillo LF (2009) Stochastic epidemic modeling. Math Stat Estim Approaches Epidemiol 1–23. http://link.springer.com/chapter/10.1007/978-90-481-2313-1_2
Greenwood PE, McDonnell MD, Ward LM (2014) Dynamics of gamma bursts in local field potentials. Neural Comput
Herrick KA, Huettmann F, Lindgren MA (2013) A global model of avian influenza prediction in wild birds: the importance of northern regions. Veterinary Res 44:42
Kim JK, Negovetich NJ (2009) Ducks: the Trojan horses of H5N1 influenza. Influ Respir Viruses 3(4):121–128
Krauss S, Walker D, Pryor S P, Niles L, Chenghong L, Hinshaw V S, Webster R G (2004) Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis (Larchmont, N.Y.) 4:177–189
Kurtz TG (1978) Strong approximation theorems for density dependent Markov chains
Kuske R, Gordillo LF, Greenwood P (2007) Sustained oscillations via coherence resonance in SIR. J Theor Biol 245(3):459–469
MATLAB (2010) Version 7.10.0 (R2010a). The MathWorks Inc., Natick
McKane AJ, Nagy JD, Newman TJ, Stefanini MO (2007) Amplified biochemical oscillations in cellular systems. J Stat Phys 128(1–2):165–191
McKane AJ, Newman TJ (2005) Predator–prey cycles from resonant amplification of demographic stochasticity. Phys Rev Lett 94(21):4
Mcnamara B (1989) Theory of stochastic resonance. Phys Rev A 39(9)
Nisbet RM, Gurney W (1982) Modelling fluctuating populations. Wiley, Hoboken
Øksendal B (2003) Stochastic differential equations. http://link.springer.com/content/pdf/10.1007/978-3-642-14394-6_5.pdf
Olsen B, Munster VJ, Wallensten A (2006) Global patterns of influenza a virus in wild birds. Science 312(5772):384–388
Roche B, Lebarbenchon C (2009) Water-borne transmission drives avian influenza dynamics in wild birds: the case of the 2005–2006 epidemics in the Camargue area. Infect Genet Evol 9(5):800–805
Rohani P, Breban R, Stallknecht DE, Drake JM (2009) Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc Nat Acad Sci 106(25):10365–10369
Sharp G, Kawaoka Y, Wright S, Turner B, Hinshaw V, Webster R (1993) Wild ducks are the reservoir for only a limited number of influenza a subtypes. Epidemiol Infect 110(01):161–176
Tomé T, de Oliveira MJ (2009) Role of noise in population dynamics cycles. Phys Rev E 79(6):061128
Tuncer N, Martcheva M (2013) Modeling seasonality in avian influenza h5n1. pp 1–27
Uhlenbeck GE, Ornstein LS (1930) On the theory of Brownian motion. Phys Rev. http://journals.aps.org/pr/abstract/10.1103/PhysRev.36.823
van der Goot J a, de Jong MC M, Koch G, Van Boven M (2003) Comparison of the transmission characteristics of low and high pathogenicity avian influenza A virus (H5N2). Epidemiol Infect 131(2):1003–1013
Van Kampen NG (1992) Stochastic processes in physics and chemistry, Vol. 11, 2nd edn, North- Holland Personal Library, Amsterdam. http://books.google.com/books?hl=en&lr=&id=3e7XbMoJzmoC&pgis=1
Wang RH, Jin Z, Liu QX, van de Koppel J, Alonso D (2012) A simple stochastic model with environmental transmission explains multi-year periodicity in outbreaks of avian flu. PLoS One 7(2):e28873
Webster RG, Bean WJ (1992) Evolution and ecology of influenza A viruses. Microbiol Rev 56(1):152–179
World Health Organization (2015) Avian influenza in humans. http://www.who.int/influenza/human_animal_interface/avian_influenza/en/. Accessed 26 Oct 2015
Acknowledgements
We gratefully acknowledge the BRAES institute at UBCO for supporting this work.
Author’s contributions MAM formulated the research problem, developed the methods of analysis, simulated and analysed the model, and drafted the manuscript; PEG and RCT evaluated the results and helped draft the manuscript. All authors gave final approval for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Appendices
Appendices
A: Derivation of the Avian Flu SDE System
First, we define the probability of a jump or an increment \(\varDelta \mathbf {X}\) as
Note that the increments of stochastic processes \(S_t,I_t\) and \(V_t\) are \(\varDelta S=S_{t+\varDelta t}-S_t\), \(\varDelta I=I_{t+\varDelta t}-I_t\), and \(\varDelta V=V_{t+\varDelta t}-V_t\), respectively. Then, the expected values of the increments given the transition probabilities in Sect. 2.1 are
Now, each increment can be expressed as the expected value of the increment plus a sum of centred increments (Greenwood and Gordillo 2009). Hence, we write the increments as:
Here, the quantities \(\varDelta Z_i\) are conditionally centred Poisson increments with mean zero with conditional variances that are related to the transition rates. The Poisson increment \(\varDelta Z_1\) corresponding to infection of a susceptible individual has a conditional variance \(\left( \beta \frac{S}{N} I + \rho S \frac{V}{N_V}\right) \varDelta t\). The increments \(\varDelta Z_2\), and \(\varDelta Z_3\) corresponding to births in susceptible class (or deaths in recovered and infected class), respectively, have conditional variances \(\mu (N-S-I) \varDelta t\), and \(\mu I \varDelta t\). On the other hand, the Poisson increment \(\varDelta Z_4\) that corresponds to recovery of an infected individual has a conditional variance equal to \(\gamma I \varDelta t\). Finally, the two increments corresponding to the replication and decay of viruses \(\varDelta Z_5\) and \(\varDelta Z_6\) must have conditional variances \(\left( \tau I +\delta V\right) \varDelta t\) and \(\eta V \varDelta t\), respectively. Divide (30) by N and \(N_V\) appropriately and take \(\varDelta t \rightarrow 0\) to obtain
Suppose we replace the Poisson increments in (30) by multiples of Wiener increments, i.e. \(\varDelta Z_i \rightarrow g_i \varDelta W_i\), with same standard deviations as the Poisson increments they replace. By doing the same limiting process \(\varDelta t \rightarrow 0\), we obtain the stochastic differential equations (SDE):
where
Furthermore, we can re-write (32) by expressing the host and virus populations as proportions rather than absolute numbers, i.e.
The corresponding SDEs for the proportions of ducks and virus are then given by (2).
The approximation (32) is an example of a result of Kurtz (1978). An alternate approach is to use a van Kampen (Van Kampen 1992) system-size expansion of the Kolmogorov (Master) equation, see e.g. in Baxendale and Greenwood (2011).
B: Stochastic Linearization
In matrix notation, (2) can be written as:
where \(\mathbf {D}=diag(\frac{1}{\sqrt{N}},\frac{1}{\sqrt{N}},\frac{1}{\sqrt{N_V}})\), \(d\mathbf {W}(t)=(dW_1, dW_2, dW_3, dW_4, dW_5, dW_6)^T,\)
and \(\mathbf {G}(\mathbf {x}(t))=\begin{bmatrix} -G_1&G_2&G_3&0&0&0 \\ G_1&0&-G_3&-G_4&0&0\\ 0&0&0&0&G_5&-G_6\end{bmatrix}.\)
Note that \(\displaystyle \mathbf {x}=(s,i,v)\) which depends on N and \(N_V\) and \(\lim _{N,N_V\rightarrow \infty }\displaystyle \mathbf {F(x)}\) is a vector whose components are the right-hand side of (4). It has been pointed out by Allen et al. (2008) that one can construct a stochastic system, which is the same in distribution such that all matrices in the diffusion term of (34) are square matrices whose sizes are equal to the dimension of \(\mathbf {x}\), i.e. in this case, a matrix \(\mathbf {C} \in \mathbb {R}^{3 \times 3}\) such that (34) would be equivalent in law to the stochastic system
The Wiener processes \({\tilde{\mathbf {W}}} \in \mathbb {R}^{3 \times 1}\) and \(\mathbf {W} \in \mathbb {R}^{6 \times 1}\) both have independent terms. Moreover, the stochastic processes \({\tilde{\mathbf {x}}}\) in (35) are different from the originally defined stochastic processes found in (34), but it can be shown that their stochastic paths are the same. Thus, \({\tilde{\mathbf {x}}}\) can be replaced by the \(\mathbb {R}^3\)-valued stochastic process \(\mathbf {x}\) that is considered originally. Matrices \(\mathbf {G}\) and \(\mathbf {C}\) are related through the \(3 \times 3\) matrix \(\mathbf {V}\), where \(\mathbf {V}=\mathbf {G}\mathbf {G}^\intercal \) and \(\displaystyle \mathbf {C}=\mathbf {V}^{1/2}\). An explicit computation of \(\mathbf {V}\) confirms that it is the general form for the noise covariance matrix \(\mathbf {B}\) that was described in Wang et al. (2012). In other words,
Letting \(N,N_V \rightarrow \infty \) so that \(s \rightarrow \phi _1, i \rightarrow \phi _2, \text {and } v \rightarrow \psi \) and \(t \rightarrow \infty \) we have \(\phi _1 \rightarrow \phi _1^*, \phi _2 \rightarrow \phi _2^*, \text {and } \psi \rightarrow \psi ^*\) implies that \(\lim _{N,N_V,t \rightarrow \infty } \mathbf {V}=\mathbf {B}\) which is a constant matrix whose entries are displayed as follows where \(\mathbf {x_{eq}}\equiv (\phi _1=\phi _1^*,\phi _2=\phi _2^*,\psi =\psi ^*)\), the equilibrium state of the deterministic system:
It remains to show that the set of Langevin equations obtained by Wang et al. (2012) can be constructed from the linear stochastic differential equations (the tilde in (35) is dropped for brevity)
Recall that the diagonal matrix \(\mathbf {D}\) is given in (34) and \(\mathbf {C}=\mathbf {V}^{1/2}\) where the entries of \(\mathbf {V}\) is described in (36). The system (38) with the stochastic term generates the deterministic process (4), since (38) becomes (4) as \(N,N_V \rightarrow \infty \) which means that this term describes the average dynamics of the processes. On the other hand, the second term is referred to as the diffusion term. It represents the variation from the average dynamics, the \(\mathcal {O}(N^{-1/2})\) fluctuations of \(\mathbf {x}(t)\) away from the deterministic process. The diffusion term prevents a damped system from settling to an equilibrium state.
We linearize (38) using the substitution \(\mathbf {x}(t)=\mathbf {x_{eq}}+\mathbf {D}{\varvec{\xi }(t)}\) and obtain
The Jacobian of \(\mathbf {F}(\mathbf {x})\) evaluated at \(\mathbf {x_{eq}}\) is denoted by \(\mathbf {J}(\mathbf {x_{eq}})\). Now, \(\mathbf {F}(\mathbf {x_{eq}})=\mathbf {0}\) and so simplifies (39), after pre-multiplying by \(\mathbf {D^{-1}}\), to
Eq. (40) is the Langevin (i.e. stochastic) equation in Wang et al. (2012)(See Eq. 6) written in slightly different form. In particular, the two equations would be equivalent if we divide (40) by dt and denote \(\mathbf {A}=\mathbf {J}(\mathbf {x_{eq}})\) and represent the diffusion term as \(\varvec{\zeta }(t)\), i.e. Gaussian white noise with correlation function \(\langle {\varvec{\zeta }(t),\varvec{\zeta }(t')^{T}}\rangle =\mathbf {B}\delta (t-t')\). In (7), we have \(\mathbf {A_0}=\mathbf {J}(\mathbf {x_{eq}})\) and \(\mathbf {C_0}=\mathbf {C}(\mathbf {x_{eq}})\).
C: Approximate Solution for Linear Diffusion Equations in Three Dimensions
We follow Baxendale and Greenwood (2011) to derive the approximate solution for our example where the diffusion processes have values in \(\mathbb {R}^3\).
Consider the stochastic system
where,
and
Here, \(\mathbf {W}(t)\) contains independent Wiener processes (or Brownian motion).
Suppose that \(\mathbf {A_0}\) has eigenvalues \(-\zeta \) and \(-\lambda \pm i\omega \) for \(\zeta ,\lambda , \omega \in \mathbb {R}^{+}\). One can find a matrix \(\mathbf {Q} \in \mathbb {R}^{3 \times 3}\) such that
The matrix \(\mathbf {\Lambda }\) is called the real block diagonal form of the eigenvalue matrix of \(\mathbf {A_0}\) so it follows that \(\mathbf {Q}\) is the real block diagonal form of the associated matrix of eigenvectors. By pre-multiplying (41) with \(\mathbf {Q}^{-1}\) and using the substitution \(\mathbf {y}(t)=\mathbf {Q}^{-1}\varvec{\xi }(t)\),we have
Let \(\mathbf {\Sigma } = \mathbf {Q}^{-1} \mathbf {C_0}\) and denote \(\mathbf {\Sigma }_{\bullet j}\) and \(\mathbf {\Sigma }_{i\bullet }\) as its jth column vector and ith row vector, respectively. With \(\mathbf {y}=[y_1,y_2,y_3]^\intercal \), we write (45) as
where \(\qquad {\tilde{\mathbf {y}}}=[y_2,y_3]^\intercal ,\)\({\tilde{\varvec{\Lambda }}}=\begin{bmatrix} -\lambda&\omega \\ -\omega&-\lambda \end{bmatrix},\) and \({\tilde{\varvec{\Sigma }}}=\left[ \mathbf {\Sigma }_{2\bullet },\mathbf {\Sigma }_{3\bullet }\right] ^\intercal .\)
Now, using the result of Allen et al. (2008), we find that the SDE (46a) is equivalent to
where \(\displaystyle \sigma _1^2=\mathbf {\Sigma _{1\bullet }}\mathbf {\Sigma ^\intercal _{1\bullet }}\) is the variance of the stationary distribution of \(y_1(t)\) and \(W_1(t)\) is a one-dimensional Wiener process. It is apparent that (47) describes an Ornstein–Uhlenbeck process (Uhlenbeck and Ornstein 1930) in one dimension with a stationary variance \(\displaystyle \sigma ^2_1/2\zeta \). The square-root of this stationary variance corresponds to the standard deviation typically observed in the process \(y_1(t)\).
On the other hand, (46b) is equivalent to:
where \(\displaystyle {\tilde{\mathbf {C}}}=({\tilde{\varvec{\Sigma }}}{\tilde{\varvec{\Sigma }}}^\intercal )^{1/2}\) and \({\tilde{\mathbf {W}}}(t)\) is a two-dimensional Wiener process. The approximate solution of (48) is related to a two-dimensional Ornstein–Uhlenbeck process as proven by Baxendale and Greenwood (2011). The approximation is reasonable under the assumption that \(\lambda \ll \omega \). Thus, if it is assumed that \(\lambda \ll \omega \) then by the theorem of Baxendale and Greenwood (2011), the approximate solution for \({\tilde{\mathbf {y}}}\) is:
where
Thus,
More precisely,
From Baxendale and Greenwood (2011), we note that (49) satisfies the stochastic equation
which was obtained by replacing \({\tilde{\mathbf {C}}}\) in (48) by \(\tilde{\sigma }\mathbf {I}\). Putting together (47) and (53), we obtain
Since \(\varvec{\xi }^{app}(t) = \mathbf {Q}\mathbf {y}^{app}(t)\), it follows from (54), using (44), that the approximate process satisfies the SDE
Now, we know that in polar coordinates
Using the formula \(x \cos {\theta } + y \sin {\theta } = z \cos ({\theta - \varphi })\) where \(z=|x+iy|\) and \(\varphi =\text {arg}(x+iy)\) for \(x,y \in \mathbb {R}\) and \(i=\sqrt{-1}\), we then write \(S_1(\lambda t)=z(\lambda t) \cos {\varphi (\lambda t)}\) and \(S_2(\lambda t)=z(\lambda t) \sin {\varphi (\lambda t)}\) with \(z(\lambda t)=\sqrt{S_1^2+S_2^2}\equiv |\mathbf {S}(\lambda t)|\) and \(\varphi (\lambda t)=\tan ^{-1}(S_2/S_1)\) to obtain
Applying (57) to the second term of (52) yields
where \(\mathbf {Q}=[q_{ij}]\).
We define \(\displaystyle q_{i2}=r_i \cos {\theta _i}\) and \(\displaystyle q_{i3}=r_i \sin {\theta _i}\) where \(r_i=\sqrt{q_{i2}^2+q_{i3}^2}\) and \(\theta _i=\tan ^{-1}(q_{13}/q_{12})\) so that the approximate fluctuation of each component takes the form:
The polar form of the approximation reveals that each model component fluctuates according to a combination of a univariate and bi-variate OU processes. The first term of the approximation contains a one-dimensional OU process weighted by a scalar determined from the transformation matrix \(\mathbf {Q}\), while the second term contains the two-dimensional OU process that varies slowly and \(\lambda t\) is a quantity that influences the radius and phase of the circular path. The stationary variance of \(\xi _i^{app}(t)\) is the sum of the stationary variance of each term in (59). This means that the long-term variance of a fluctuation is
Hence, the typical magnitude of \(\xi _i^{app}(t)\), i.e. stationary standard deviation, is
Note that the fluctuation of each component i has a constant phase shift \(\theta _i\), which is useful in computing phase differences between disease components.
D: Additional Insight From the Approximation on the Interaction of Disease Components
In Sect. 4.1, we showed that for the given set of avian flu parameter values, the system exhibits noise-sustained oscillations which can be viewed as a sum of two processes given by (24): (i) a process proportional to the one-dimensional OU process and (ii) a process proportional to the product of a rotation matrix and a standard OU process.
We describe here, using (11), the behaviour of the sample path in three-dimensional space. The first term of (11) means that sample path behaves as an Ornstein–Uhlenbeck process \(y_1(t)\) that travels along the axis that points to the direction of \(\mathbf {Q_{\bullet 1}}\), i.e. the eigenvector associated with \(-\zeta \). In addition, the second term of (11) implies that the sample path cycles on the subspace spanned by the last two column vectors of the transformation matrix \(\mathbf {Q}\), i.e. eigenvectors of the eigenvalues \(-\lambda \pm i\omega \). This subspace contains a plane whose equation (see (66) in Appendix E for general formulation), for our chosen set of parameters, is given by:
Figure 10 shows the plane (62) and a realization of the stochastic simulation of (24). In Fig. 10a, we observe that the sample path lies chiefly on or near the plane (62). However, if we neglect the first term of (24), the dynamics of the fluctuations lie entirely on this plane (see Fig. 10b). Thus, the portion of the sample path that departs from the plane is clearly due to the one-dimensional OU process whereas the second term constrains the sample path to move within the plane.
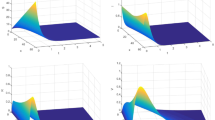
From (24), we know that the stationary standard deviation of \(y_1(t)\) is 1.63 which is small compared to the constant \(\tilde{\sigma }/\sqrt{\lambda }=10.21\) that appears in the second term of the equation. Therefore, we can neglect the first term of (24) and show that avian flu epidemics cycle on the plane (62). This can be achieved mathematically when the magnitude of the real eigenvalue \(\zeta \) is larger than \(\lambda \), which means that the approximate process approaches the hyperplane in fast manner. In Fig. 11, we compute the magnitude \(\zeta \) over combinations of \(\beta \) and \(\rho \) values and found that the epidemic cycles occur primarily in the hyperplane when \(\beta \) is below 100 and \(\rho \) is high, i.e. where \(\zeta \) is larger than \(\lambda \).
The fact that avian flu dynamics could primarily occur in the plane suggest that under certain conditions for each transmission route, one can project the avian flu system (7) onto the plane (62) and so simplify the analysis. For instance, using the equation of the plane, we can write one component in terms of the other and convert the three-dimensional linear avian flu SDE system (7) into a two-dimensional one. The possibility of modelling recurrent avian flu epidemics as stochastic system in two dimensions must therefore be explored.
Plot of \(\zeta \) (left panel) and \(\lambda \) (right panel) as functions of \(\beta \) and \(\rho \). The white region is where \(R_0<1\), i.e. noise-sustained oscillations cannot be observed here. Default parameter values are in Table 1 (Color figure online)
E: The Subspace Where the Cycling Takes Place
For the case when the stationary standard deviation (s.s.d.) of the second term is very large compared to the s.s.d. of the first term in our approximation, the first term of (52) is negligible and we expect the stochastic path to lie near a plane, i.e. a subspace of \(\mathbb {R}^3\), spanned by the last two column vectors of \(\mathbf {Q}\) (\(\mathbf {Q_{\bullet 2}}\) and \(\mathbf {Q_{\bullet 3}}\)). Here, we show a general way for computing the equation of this plane.
The sample path defined by the fluctuations \(\xi _i(t)\) is centred at (0, 0, 0) and so the equation of the plane should take the form:
We know that the vectors \(\mathbf {Q_{\bullet 2}}\) and \(\mathbf {Q_{\bullet 3}}\) span the plane, hence must satisfy (63). Therefore,
By Gaussian elimination or by manipulating the explicit form of the linear system, we can eliminate \(a_3\) in (64) and a little algebra turns (64) into a simpler equation,
Now we require \(\det (\mathbf {M_2}) \ne 0\) so that \(\displaystyle a_2 = -\frac{\det (\mathbf {M_1})}{\det (\mathbf {M_2})}a_1\), which gives \(\displaystyle a_3=\frac{a_1}{q_{32}}\left( \frac{\det (\mathbf {M_1})}{\det (\mathbf {M_2})}q_{22}-q_{12}\right) .\) Therefore, assuming that \(a_1 \ne 0\), the desired equation of the plane is
F: Derivation of the Explicit Form of the Mean-Field Eigenvalues
Our starting point is the Jacobian evaluated at the stable endemic equilibrium point (Wang et al. 2012), i.e.
where \(\displaystyle \phi _1^*=\frac{1}{\mathcal {R}_0}\), \(\displaystyle \phi _2^*=\frac{\mu }{\mu +\gamma }\left( 1-\frac{1}{\mathcal {R}_0}\right) \), and \(\displaystyle \psi ^{*}=\frac{\kappa \mu \tau }{(\eta -\delta )(\mu +\gamma )}\left( 1-\frac{1}{\mathcal {R}_0}\right) \) for the basic reproduction number
The condition \(\mathcal {R}_0>1\) must be satisfied for the non-trivial steady-state \(\mathbf {x_{eq}}=(\phi _1^{*},\phi _2^{*},\psi ^{*})\) to exist.
The eigenvalues of \(\mathbf {J}(\mathbf {x_{eq}})\) determine the local dynamics of the deterministic SIR-V system close to the non-trivial equilibrium point \(\mathbf {x_{eq}}\). Now, denote the eigenvalues of \(\mathbf {J}(\mathbf {x_{eq}})\) as \(\nu \). It follows that \(|\mathbf {J}(\mathbf {x_eq})-\nu I|=0\) gives rise to a cubic polynomial of the form
where:
Equations (68) and (69) in fact appeared in the Appendix section of Wang et al. (2012), where it was proven that the endemic equilibrium is stable. Now, substitute \(\displaystyle \nu =y+\frac{a}{3}\) to yield the normal form transformation,
This method is also known as Vieta’s substitution Connor (1956). Equation (70) has been well-studied and has known solutions in general form:
where: \(\displaystyle Y_{\pm }=\left( -\frac{q}{2}\pm \sqrt{\frac{q^2}{4}+\frac{p^3}{27}}\right) ^{1/3}\) and \(i=\sqrt{-1}\).
We are interested in the case when all three roots exist with two of them being complex conjugates. This is satisfied by assuming that \(\displaystyle \frac{q^2}{4} + \frac{p^3}{27}>0\).
By back substitution, the solutions of (68) are:
The eigenvalues \(\nu _2\) and \(\nu _3\) are conjugate pairs whose real part is negative as confirmed by Wang et al. (2012). The magnitude of the real and imaginary part corresponds to the decay rate \(\lambda \) and the intrinsic frequency \(\omega \), respectively, of the deterministic system linearized at the endemic equilibrium state. Therefore,
Using avian flu parameters in Table 1, we can write \(\lambda \) and \(\omega \) in terms of \(\beta \) and \(\rho \), as follows:
where
Here
and
Additionally, the first eigenvalue \(\nu _1<0\) (eigenvalue with largest negative real part) and so the decay rate of the OU process \(y_1(t)\) in (11) is \(\zeta =|\nu _1|\).
Rights and permissions
About this article
Cite this article
Mata, M.A., Greenwood, P. & Tyson, R. The Relative Contribution of Direct and Environmental Transmission Routes in Stochastic Avian Flu Epidemic Recurrence: An Approximate Analysis. Bull Math Biol 81, 4484–4517 (2019). https://doi.org/10.1007/s11538-018-0414-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-0414-6