Abstract
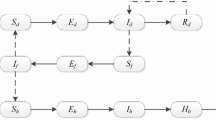
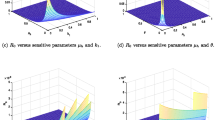
We propose and analyze a mathematical model of a vector-borne disease that includes vector feeding preference for carrier hosts and intrinsic incubation in hosts. Analysis of the model reveals the following novel results. We show theoretically and numerically that vector feeding preference for carrier hosts plays an important role for the existence of both the endemic equilibria and backward bifurcation when the basic reproduction number \({\mathcal {R}}_0\) is less than one. Moreover, by increasing the vector feeding preference value, backward bifurcation is eliminated and endemic equilibria for hosts and vectors are diminished. Therefore, the vector protects itself and this benefits the host. As an example of these phenomena, we present a case of Andean cutaneous leishmaniasis in Peru. We use parameter values from previous studies, primarily from Peru to introduce bifurcation diagrams and compute global sensitivity of \({\mathcal {R}}_0\) in order to quantify and understand the effects of the important parameters of our model. Global sensitivity analysis via partial rank correlation coefficient shows that \({\mathcal {R}}_0\) is highly sensitive to both sandflies feeding preference and mortality rate of sandflies.







Similar content being viewed by others
References
Abboubakar H, Buonomo B, Chitnis N (2016) Modelling the effects of malaria infection on mosquito biting behaviour and attractiveness of humans. Ricerche di matematica 65(1):329–346
Barradas I, Caja Rivera RM (2018) Cutaneous leishmaniasis in Peru using a vector-host model: backward bifurcation and sensitivity analysis. Math Methods Appl Sci 41:1908–1924
Biswas D, Datta A, Roy PK (2016a) Combating leishmaniasis through awareness campaigning: a mathematical study on media efficiency. Int J Math Eng Manag Sci 1(3):139–149
Biswas D, Roy PK, Li XZ, Basir FA, Pal J (2016b) Role of macrophage in the disease dynamics of cutaneous Leishmaniasis: a delay induced mathematical study. Commun Math Biol Neurosci, Article ID, p 4
Biswas D, Kesh DK, Datta A, Chatterjee AN, Roy PK (2014) A mathematical approach to control cutaneous leishmaniasis through insecticide spraying. Sop Trans Appl Math 1(2):44–54
Buonomo B, Vargas-De-Leon C (2013) Stability and bifurcation analysis of a vector-bias model of malaria transmission. Math Biosci 242(1):59–67
Cáceres AG et al (2004) Epidemiology of Andean cutaneous leishmaniasis: incrimination of Lutzomyia ayacuchensis (Diptera: Psychodidae) as a vector of Leishmania in geographically isolated, upland valleys of Peru. Am J Trop Med Hyg 70(6):607–612
Caja R (2018) Control policies and vector behavioral effects in modeling vector–host interactions: novel applications for cutaneous leishmaniasis in Peru. Ph.D. thesis, CIMAT, Research Center in Mathematics at Guanajuato-Mexico, Department of Mathematics
Castillo-Chavez BS (2004) Dynamical models of tuberculosis and their applications. Math Biosci Eng 1(2):361–404
C.D.C. (Center for Disease Control and Prevention) (2013) Parasites-leishmaniasis
Chamchod F, Britton NF (2011) Analysis of a vector-bias model on malaria transmission. Bull Math Biol 73(3):639–657
Coleman RE, Edman JD (1988) Feeding-site selection of Lutzomyia longipalpis (Diptera: Psychodidae) on mice infected with Leishmania mexicana amazonensis. J Med Entomol 25(4):229–233
Diekmann O, Heesterbeek JAP, Metz JA (1990) On the definition and the computation of the basic reproduction ratio \(R_0\) in models for infectious diseases in heterogeneous populations. J Math Biol 28(4):365–382
Dujardin JC, Llanos-Cuentas A, Caceres A, Arana M, Dujardin JP, Guerrini F, Hamers R (1993) Molecular karyotype variation in Leishmania (Viannia) peruviana: indication of geographical populations in Peru distributed along a north-south cline. Ann Trop Med Parasitol 87(4):335–347
Dushoff J, Huang W, Castillo-Chavez C (1998) Backwards bifurcations and catastrophe in simple models of fatal diseases. J Math Biol 36(3):227–248
Gorahava K, Rosenberger JM, Mubayi A (2015) Optimizing insecticide allocation strategies based on houses and livestock shelters for visceral leishmaniasis control in Bihar. India. Am J Trop Med Hygiene 93(1):114–122
INEI (Peruvian National Institute of Statistics and Informatics). Peruvian population 1995–2015
Knols BG, Meuerink J (1997) Odors influence mosquito behavior. Sci Med 4:56–63
Koella JC et al (1998) The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc R Soc Lond B Biol Sci 265(1398):763–768
Lainson R, Shaw JJ (1978) Epidemiology and ecology of leishmaniasis in Latin-America. Nature 273:595–600
Lemon, Stanley M et al (2008) Vector-borne diseases: understanding the environmental, human health, and ecological connections. Workshop summary. En Vector-borne diseases: understanding the environmental, human health, and ecological connections. Workshop summary. National Academies Press
Leonardo S et al (2004) Leishmaniasis. Peruvian. J Dermatol 14(2):82–98
Lyimo IN, Ferguson HM (2009) Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol 25(4):189–196
Llanos-Cuentas EA, Davies CR, Pyke SDM, Dye C (1995) Cutaneous leishmaniasis in the Peruvian Andes: an epidemiological study of infection and immunity. Epidemiol Infect 114(02):297–318
Marino S, Hogue IB, Ray CJ, Kirschner DE (2008) A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theoret Biol 254(1):178–196
Ministry of Health of Peru (MINSA) (2015) Bulletin of Epidemiology 2013, 2014, 2015
McKay MD, Beckman RJ, Conover WJ (1979) Comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 21(2):239–245
Moore J (1995) The behavior of parasitized animals. Bioscience 45(2):89–96
ORegan SM, Lillie JW, Drake JM (2016) Leading indicators of mosquito-borne disease elimination. Theor Ecol 9(3):269–286
O’Shea B, Rebollar-Tellez E, Ward RD, Hamilton JGC, El Naiem D, Polwart A (2002) Enhanced sandfly attraction to Leishmania-infected hosts. Trans R Soc Trop Med Hyg 96(2):117–118
Pan American Health Organization-World Health Organization (2014) Leishmaniasis: small bites big threats
Pedro SA, Tonnang HEZ, Abelman S (2016) Uncertainty and sensitivity analysis of a Rift Valley fever model. Appl Math Comput 279:170–186
Pérez JE, Ogusuku E, Inga R, Lopez M, Monje J, Paz L, Guerra H (1994) Natural Leishmania infection of Lutzomyia spp. in Peru. Trans R Soc Trop Med Hyg 88(2):161–164
Penn D, Potts WK (1998) Chemical signals and parasite-mediated sexual selection. Trends Ecol Evolut 13(10):391–396
Rabinovich JE, Feliciangeli MD (2004) Parameters of Leishmania braziliensis transmission by indoor Lutzomyia ovallesi in Venezuela. Am J Trop Med Hyg 70(4):373–382
Rogers ME, Bates PA (2007) Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathog 3(6):818–826
Takken W, Verhulst NO (2013) Host preferences of blood-feeding mosquitoes. Annu Review Entomol 58:433–453
The Center for Food Security and Public Health (2009) Leishmaniasis (Cutaneous and Visceral)
Van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180(1):29–48
Villaseca P, Llanos-Cuentas A, Perez E (1993) A comparative field study of the relative importance of Lutzomyia peruensis and Lutzomyia verrucarum as vectors of cutaneous leishmaniasis in the Peruvian Andes. Am J Trop Med Hyg 49(2):260–269
Villavicencio Pulido G, Barradas I, Luna B (2016) Backward bifurcation for some general recovery functions. Mathematical methods in the applied sciences. Addison-Wesley, Boston
Votypka J, Pruzinova K, Hlavacova J, Volf P (2015) The effect of temperature and avian blood on Leishmania development in sand flies bulletin of SEA, vol 26
W.H.O. (2016) Vector-borne diseases. http://www.who.int/mediacentre/factsheets/fs387/en/
Zheng Y, Rundell A (2006) Comparative study of parameter sensitivity analyses of the TCR-activated Erk-MAPK signalling pathway. IEE Proc Syst Biol 153(4):201–211
Acknowledgements
Rocío Marilyn Caja Rivera acknowledges fruitful conversations to Dr. Linda Allen (TEXAS TECH UNIVERSITY), Dr. Abraham Cáceres (UNMSM-PERU), Dr. Sergio Ibañez (INECOL-MEXICO) and she expresses gratefulness to anonymous reviewers for careful reading and valuable comments to this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Proof of Theorem 1
1.a)
As \(f(\lambda _h)\) in Eq. (10) is a quadratic function with \(A>0\), it follows that the minimum of f occurs at \(\hat{\lambda }_h= -\frac{B}{2A}>0\) with \(f(\hat{\lambda _h})=-\frac{B^{2}-4AC}{4A}\). Thus if \(B^2-4AC=0\), then system (1) has a unique endemic equilibrium.
1.b)
As \(f(\lambda _h)\) in Eq. (10) is a quadratic function with \(B<0\), \(A>0\), \(C>0\), \(B^{2}-4AC>0\), it follows that the minimum of f occurs at \(\hat{\lambda }_h= -\frac{B}{2A}>0\) with \(f(\hat{\lambda _h})=-\frac{B^{2}-4AC}{4A}\). Thus if \(B^{2}-4AC>0\), then system (1) has two endemic equilibria.
1.c)
For \(\alpha _v<\alpha _v^{*}\) and \({\mathcal {R}}_{0}<\sqrt{H}<1\). By hypothesis, \(A>0\), \(B>0\) and \(C>0\). Then, Eq. (10) does not have any positive root. Thus, conclusion 1.c) holds.
1.d)
For \(\alpha _v<\alpha _v^{*}\) and \({\mathcal {R}}_{0} \ge 1\) we get \(B<0\) and \(C\le 0\). Then conclusion 1.d holds.
2.a)
For \(\alpha _v \ge \alpha _v^{*}\) and \({\mathcal {R}}_{0} >1\) we get \(C<0\). Then system (1) has a unique endemic equilibrium.
2.b)
For \(\alpha _v \ge \alpha _v^{*}\) and \({\mathcal {R}}_{0} \le 1\) we get \(C\ge 0\) and \(H>{\mathcal {R}}_{0}^2\). Then system (1) has no endemic equilibrium. \(\square \)
Appendix 2
Proof of Theorem 2
The Jacobian matrix of system (1), computed at \(E_{0}\) for \(b_1^*\), is given by :
The characteristic polynomial of the Jacobian matrix is:
where
We replace the value of \(b_1^*\) in \(d_3\), and we get
The Jacobian matrix admits a zero eigenvalue and the other eigenvalues are real and negative. Thus, the disease-free equilibrium \(E_0\) is a non-hyperbolic equilibrium and assumption (A1) of Theorem (Castillo-Chavez 2004) is demonstrated. We indicate by \(v=(v_1, v_2, v_3,v_4,v_5)\) and \(w=(w_1,w_2,w_3,w_4,w_5)^T\), a right and a left eigenvector associated with the zero eigenvalue, respectively, such that their dot product is one \(v.w=1\). Multiplying vJ and Jw and setting each of them equal to zero yields:
then,
where
The functions \(f_k\), \(k=1,\ldots ,5\) are the right side of the differential equations in (1a)-(1e). We define two quantities important for verification of the subcritical bifurcation
It can be checked that:
Accordingly to the coefficients a and b described in Theorem 4.1 of (Castillo-Chavez 2004), it follows:
and
Then, a is positive when \(\alpha _v<\frac{\mu _v((\mu _h+\delta _h)(\mu _h+\sigma _h)+\mu _h\omega _h)}{\mu _h\sigma _h(2\mu _v+b_2\beta _2)} =\alpha _v^*\). Consequently, system (1) shows backward bifurcation at \({\mathcal {R}}_0\) when \(\alpha _v< \alpha _v^*\).
On the other hand, a is always negative when
Therefore, system (1) exhibits a forward bifurcation at \({\mathcal {R}}_{0}=1\) when \(\alpha _v>\alpha _v^*\). \(\square \)
Rights and permissions
About this article
Cite this article
Caja Rivera, R., Barradas, I. Vector Preference Annihilates Backward Bifurcation and Reduces Endemicity. Bull Math Biol 81, 4447–4469 (2019). https://doi.org/10.1007/s11538-018-00561-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-00561-1