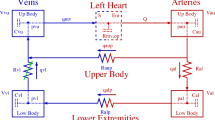
Abstract
In this paper, we develop a pulsatile model for the cardiovascular system which describes the reaction of this system to a submaximal constant workload imposed on a person at a bicycle ergometer test after a period of rest. Furthermore, the model should allow to use measurements for the pulsatile pressure in fingertips which provide information on the diastolic and the systolic pressure for parameter estimation. Based on the assumption that the baroreceptor loop is the essential control loop in this case, we design a stabilizing feedback control for the pulsatile model which is obtained by solving a linear-quadratic regulator problem for the linearization of a non-pulsatile counterpart of the pulsatile model. We also investigate the behavior of the model with respect to changes in the weight of the term in the cost functional for the linear-quadratic regulator problem which penalizes the deviation of the momentary pressure in the aorta from the pressure at the stationary situation which should be obtained.







Similar content being viewed by others
References
Arechavaleta G, Laumond JP, Hicheur H, Berthoz A (2008) An optimality principle governing human walking. IEEE Trans Robot 24(1):5–14
Batzel JJ, Kappel F, Timischl-Teschl S (2005) A cardiovascular-respiratory control system model including state delay with application to congestive heart failure in humans. J Math Biol 50:293–335
Batzel JJ, Kappel F, Schneditz D, Tran HT (2007) Cardiovascular and respiratory systems: modeling, analysis and control, frontiers in applied mathematics, vol 34. SIAM, Philadelphia
Bazett HC (1920) An analysis of the time-relations of electrocardiograms. Heart 7:353–370
Bowditch HP (1871) Über die Eigenthümlichkeiten der Reizbarkeit, welche die Muskelfasern des Herzens zeigen. Berichte der Königlichen Sächsischen Gesellschaft der Wissenschaften, Mathematisch-Physische Classe, Sitzung am 12 Dezember 1871 23:651–689
Danielsen M (1998) Modeling of feedback mechanisms which control the heart function in a view to an implementation in cardiovascular models. PhD thesis, Roskilde University
Danielsen M, Ottesen JT (1997) A dynamical approach to the baroreceptor regulation of the cardiovascular system. In: Proceeding of the 5th International Symposium, Symbiosis 1997, pp 25–29
Danielsen M, Ottesen JT (2001) Describing the pumping heart as a pressure source. J Theor Biol 212(1):71–81
de los Reyes VAA (2010) A mathematical model for the cardiovascular system with a measurable pulsatile pressure output. PhD thesis, University of Graz, Institute for Mathematics and Scientific Computing
de los Reyes VAA, Kappel F (2010a) A mathematical cardiovascular model with pulsatile and non-pulsatile components. Tech. Rep. 011, Spezialforschungsbereich F32, University of Graz
de los Reyes VAA, Kappel F (2010b) Modeling pulsatility in the human cardiovascular system. Mathematica Balkanica (New Series) 24(3—4):229–242. In: Proceedings of the “SEE Young Researchers Workshop”, MASSEE International Congress on Mathematics - MICOM 2009, September 16–20, 2009, Ohrid, Macedonia
Doubek E (1978) Least energy regulation of the arterial system. Bull Math Biol 40:79–93
Ellwein LM, Tran HT, Zapata C, Novak V, Olufsen MS (2008) Sensitivity analysis and model assessment: mathematical models for arterial blood flow and blood pressure. Cardiovasc Eng 8:94–108
Fink M, Batzel JJ, Kappel F (2004) An optimal control approach to modeling the cardiovascular-respiratory system: an application to orthostatic stress. Cardiovasc Eng 4(1):27–38
Fister KR, McCarthy CM (2003) Optimal control of a chemotaxis system. Q Appl Math 61(2):193–211
Gompertz B (1825) On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos Trans R Soc London 115:513–583
Grodins FS (1959) Integrative cardiovascular physiology: a mathematical synthesis of cardiac and blood vessel hemodynamics. Q Rev Biol 34(2):93–116
Grodins FS (1963) Control theory and biological systems. Columbia University Press, New York
Gusev S, Johansson S, Kågström B, Shiriaev A, Varga A (2009) A numerical evaluation of solvers for the periodic Riccati differential equation. Tech. Rep. Report / UMINF 09.03, Institutionen för Datavetenskap, Umeå Universitet
Guyton A, Hall J (2006) Textbook of medical physiology, 11th edn. Elsevier, Amsterdam
Heldt T, Shim EB, Kamm RD, Mark RG (2002) Computational modeling of cardiovascular response to orthostatic stress. J Appl Physiol 92:1239–1254
Huntsman LL, Noordergraaf A, Attinger EO (1978) Metabolic autoregulation of blood flow in skeletal muscle: a model. In: Baan J, Noordergraaf A, Raines J (eds) Cardiovascular system dynamics. MIT Press, Cambridge, pp 400–414
Janssen PML (2010) Myocardial contraction-relaxation coupling (54th Bowditch Lecture). Am J Physiol Heart Circ Physiol 299(6):H1741–H1749. doi:10.1152/ajpheart.00759.2010
Johansson S, Kågström B, Shiriaev A, Varga A (2007) Comparing one-shot and multishot methods for solving periodic riccati differential equations. In: Proceedings of the 3rd IFAC Workshop on periodic control systems, PSYCO07, St. Petersburg, Russia
Kappel F (2012) Modeling the dynamics of the cardiovascular-respiratory system (CVRS) in humans, a survey. Math Model Nat Pheno 7:65–77
Kappel F, Peer RO (1993) A mathematical model for fundamental regulation processes in the cardiovascular system. J Math Biol 31:611–631
Kappel F, Fink M, Batzel JJ (2007) Aspects of control of the cardiovascular-respiratory system during orthostatic stress induced by lower body negative pressure. Math Biosci 206(2):273–308
Kenner T, Pfeiffer KP (1980) Studies on the optimal matching between heart and arterial system. In: Baan J, Arntzenius AC, Yellin EL (eds) Cardiac dynamics, developments in cardiovascular medicine, vol 2. Martinus Nijhoff Publishers bv, The Hague, pp 261–270
Klabunde RE (2011) Cardiovascular physiology concepts, 2nd edn. Lippincott Williams & Wilkins, Philadelphia
Křivan V (1996) Optimal foraging and predator-prey dynamics. Theor Popul Biol 49(3):265–290
Kwakernaak H, Sivan R (1972) Linear optimal control systems. Wiley-Interscience, Hoboken
Lebiedz D, Maurer H (2004) External optimal control of self-organisation dynamics in a chemotaxis reaction diffusion system. Syst Biol 1(2):222–229
Lenhart S, Workman JT (2007) Optimal control applied to biological models. Chapman and Hall/CRC, London
Noldus EJ (1976) Optimal control aspects of left ventricular ejection dynamics. J Theor Biol 63(2):275–309
Noordergraaf A (1969) Hemodynamics in biological engineering. McGraw-Hill, New York
Olufsen MS, Ottesen JT (2013) A practical approach to parameter estimation applied to model predicting heart rate regulation. J Math Biol 67(1):39–68. doi:10.1007/s00285-012-0535-8
Olufsen MS, Tran HT, Ottesen JT (2004) Modeling cerebral blood flow control during posture change from sitting to standing. Cardiovasc Eng 4:47–58
Olufsen MS, Ottesen JT, Tran HT, Ellwein LM, Lipsitz LA, Novak V (2005) Blood pressure and blood flow variation during postural change from sitting to standing: model development and validation. J Appl Physiol 99(4):1523–1537
Olufsen MS, Tran HT, Ottesen JT, Experiences Research, for Undergraduates Program, Lipsitz LA, Novak V, (2006) Modeling baroreflex regulation of heart rate during orthostatic stress. Am J Physiol Regul Integr Comp Physiol 291(5):R1355b–R1368
Olufsen MS, Alston AV, Tran HT, Ottesen JT, Novak V (2008) Modeling heart rate regulation—Part I: sit-to-stand versus head-up tilt. Cardiovasc Eng 8(2):73–87
Ono K, Uozumi T, Yoshimoto C, Kenner T (1982) The optimal cardiovascular regulation of the arterial blood pressure. In: Kenner T, Busse R, Hinghofer-Szalkay H (eds) Cardiovascular system dynamics: models and measurements. Plenum Press, New York, pp 119–139
Ottesen JT (1997) Modelling of the baroreflex-feedback mechanism with time-delay. J Math Biol 36:41–63
Ottesen JT, Danielsen M (2003) Modeling ventricular contraction with heart rate changes. J Theor Biol 222(3):337–346
Ottesen JT, Olufsen MS (2011) Functionality of the baroreceptor nerves in heart rate regulation. Comput Methods Programs Biomed 101(2):208–219
Ottesen JT, Olufsen MS, Larsen JK (eds) (2004) Applied mathematical models in human physiology. SIAM Monographs on Mathematical Modeling and Computation, SIAM, Philadelphia
Palladino JL, Noordergraaf A (2002) A paradigm for quantifying ventricular contraction. Cell Mol Biol Lett 7(2):331–335
Peskin CS (1981) Mathematical aspects of physiology. In: Hoppenstaedt F (ed) Mathematical aspects of physiology. Lectures in Applied Mathematics, vol 19. American Mathematical Society, Providence, pp 69–93
Pfeiffer K, Kenner T (1981) On the optimal strategy of cardiac ejection. In: Kenner T, Busse R, Hinghofer-Szalkay H (eds) Cardiovascular system dynamics: models and measurements. Plenum Press, New York, pp 133–136
Pope SR, Ellwein LM, Zapata C, Novak V, Kelley CT, Olufsen MS (2009) Estimation and identification of parameters in a lumped cerebrovascular model. Math Biosci Eng 6(1):93–115
Rhoades RA, Tanner GA (eds) (2003) Medical physiology, 2nd edn. Medical Physiology (Rhoades) Series, Lippincott Willliams & Wilkins, Philadelphia
Russell DL (1979) Mathematics of finite-dimensional control systems: theory and design. Marcel Dekker, New York
Sontag ED (1998) Mathematical control theory: deterministic finite dimensional systems. Textbooks in Applied Mathematics, vol 6, 2nd edn. Springer, New York
Sunagawa K, Sagawa K (1982) Models of ventricular contraction based on time-varying elastance. Crit Rev Biomed Eng 7(3):193–228
Swan G (1984) Applications of optimal control theory in biomedicine. Marcel Dekker, New York
Timischl S (1998) A global model of the cardiovascular and respiratory system. PhD thesis, University of Graz, Institute for Mathematics and Scientific Computing
Todorov E, Jordan MI (2002) Optimal feedback control as a theory of motor coordination. Nat Neurosci 5(11):1226–1235
Todorov E, Li W (2003) Optimal control methods suitable for biomechanical systems. In: Proceedings of the 25th Annual International Conference of the IEEE Engineering in Biology and Medicine Society, vol 2, September 2003 Cancun, Mexico pp 1758–1761
Ursino M (1998) Interaction between carotid baroregulation and the pulsating heart: a mathematical model. Am J Physiol 275(5):H1733–H1747
Ursino M (1999) A mathematical model of the carotid baroregulation in pulsating conditions. IEEE Trans Biomed Eng 46(4):382–392
Ursino M, Fiorenzi A, Belardinelli E (1996) The role of pressure pulsatility in the carotid baroreflex control: a computer simulation study. Comput Biol Med 26(4):297–314
Westerhof N, Stergiopulos N, Noble MIM (2005) Snapshots of hemodynamics, basic science for the cardiologist, vol 18. Springer, New York
Zabczyk J (2007) Mathematical control theory: an introduction. Modern Birkhäuser Classics, Birkhäuser, Basel
Acknowledgments
Jung’s work was supported by Konkuk University Research Grant in 2012. A. de los Reyes was supported by an ASEA-UNINET PhD-Technology Grant (administered by the Austrian Academic Exchange Service (OeAD)), by the University of the Philippines and by Konkuk University.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Parameter Values used in the Model
The following tables provide the parameter names, meanings, and values used in the simulation. The values listed in Tables 1 and 2 are taken from the literature (Batzel et al. 2007; Kappel and Peer 1993) with some modifications for the parameters of the finger compartment. The associated units are also given.
For the computation of the feedback control law, we need constant approximations \(x^{\text {rest}}\) and \(x^{\text {exer}}\) of the solutions which correspond to the stationary phases “rest” and “exercise” (see Sect. 3). This is done in Appendix 2 using the corresponding workload \(W^{\text {equil}}\), the corresponding value \(H^{\text {equil}}\) of the heart frequency and the corresponding value \(A_{\text {pesk}}^{\text {equil}}\) of Peskin’s constant in Eq. (11). In Table 2, we list the values for \(H^{\text {equil}},\, W^{\text {equil}}\), and \(A_{\text {pesk}}^{\text {equil}}\).
Appendix 2: Determination of Equilibria
The feedback law (27) for the pulsatile model (15) obtained in Section 3 requires the vector \(\bigl ((x^{\text {exer}})^{\mathsf{T}},S_{\ell \text {v}}^{\text {exer}},\sigma _{\ell \text {v}}^{\text {exer}}\bigr )^{\mathsf{T}}\). Furthermore, we also need the vector \(\bigl ((x^{\text {rest}})^{\mathsf{T}},\) \(S_{\ell \text {v}}^{\text {rest}},\sigma _{\ell \text {v}}^{\text {rest}}\bigr )^{\mathsf{T}}\) as initial condition for the model (15) and (28). In contrast to the non-pulsatile model where \({\tilde{x}}^{\text {rest}}\) and \({\tilde{x}}^{\text {exer}}\) are equilibrium solutions corresponding to the stationary situations “rest” and “exercise,” we have periodic solutions of the pulsatile model which characterize the stationary situations “rest” and “exercise.” This can be seen by numerical computations over sufficiently long-time intervals. Let the exponent “equil” stand for “rest,” respectively, for “exer”. In order to compute \(x^{\text {equil}}\), we fix \(H(t)\equiv H^{\text {equil}},\, W=W^{\text {equil}}\), and \(A_{\text {pesk}}=A_{\text {pesk}}^{\text {equil}}\) (as given in Table 2). Moreover, we also fix \(S_{\text {rv}}(t)\equiv (\beta _{\text {rv}}/\alpha _{\text {rv}})H^{\text {equil}},\, \sigma _{\text {rv}}(t)\equiv 0\), and \(S_{\ell \text {v}}(t)\equiv (\beta _{\ell \text {v}}/\alpha _{\ell \text {v}})H^{\text {equil}},\, \sigma _{\ell \text {v}}(t)\equiv 0\). Then, we compute the solution of system (15) with \(u(t)\equiv 0\) and given initial values where \(S_{\text {rv}}(0)=S_{\text {rv}}^{\text {equil}}\), and \(\sigma _{\text {rv}}(0)=0\). Note that the equations involving \(S_{\text {rv}},\, \sigma _{\text {rv}},\, S_{\ell \text {v}}\) and \(\sigma _{\ell \text {v}}\) are automatically satisfied. For the various pressures \(P_{\dots }\), we take the mean values according to the following formula (see Klabunde 2011):
where \(P_{\dots ,\text {diast}}\) and \(P_{\dots ,\text {syst}}\) are the end-diastolic and end-systolic pressures, respectively, taken at the last heart period computed. Of course, \(P_{\text {vp}}(t)\) is computed using (14). For the resistance \(R_{\text {sp}}^{\text {equil}}\), we just take the average of the values for \(R_{\text {sp}}(t)\) over the last heart period computed (there are hardly any oscillations visible in the function \(R_{\text {sp}}(t)\)). Table 3 presents the initial values chosen for the computations and the values for \(x^{\text {equil}},\, S_{\ell \text {v}}^{\text {equil}},\, \sigma _{\ell \text {v}}^{\text {equil}}\). The values obtained for the stationary phase “rest” are used as initial values for the computations in case of the phase “exercise” with the exception of \(P_{\ell \text {v}},\, S_{\text {rv}},\, \sigma _{\text {rv}},\,S_{\ell \text {v}}\), and \(\sigma _{\ell \text {v}}\).
Appendix 3: The Linear-Quadratic Regulator Problem
For the convenience of the reader, we formulate in this appendix the autonomous linear-quadratic regulator problem (LQRP) with infinite time horizon and the main result for this problem. For details, we refer to Kwakernaak and Sivan (1972); Lenhart and Workman (2007); Sontag (1998); Zabczyk (2007). The component “linear” refers to the fact that a linear control system is given, i.e., we have the following equations:
where \(x(t)\in \hbox {I}\!\hbox {R}^n,\, u(t)\in \hbox {I}\!\hbox {R}^m,\, y(t)\in \hbox {I}\!\hbox {R}^k\), and \(\mathcal {A},\, \mathcal {B},\, \mathcal {C}\) are constant matrices of appropriate dimension and \(u(\cdot )\in L^2(0,\infty ;\hbox {I}\!\hbox {R}^m)\). We denote the solution of (30) by \(x(t)=\bigl (x(t;x_0,u(\cdot )\bigr )\) and define the quadratic cost functional \(J\) by
where \(y(t)=\mathcal {C}x\bigl (t;x_0,u(\cdot )\bigr ),\, t\ge 0\), and \(R\in \hbox {I}\!\hbox {R}^{m\times m}\) is a positive definite matrix. The component “quadratic” in LQRP refers to the fact that we seek a function \(\hat{u}(\cdot )\in L^2(0,\infty ;\hbox {I}\!\hbox {R}^m)\) such that the quadratic cost functional \(J(u(\cdot ))\) is minimized, i.e.,
From control theory (see for instance, Kwakernaak and Sivan 1972), it follows that under some conditions on the matrices \(\mathcal {A},\, \mathcal {B}\), and \(\mathcal {C}\), the solution of the infinite time horizon LQRP is given by a linear, time-independent feedback law,
where \(x(t)\) is the solution of the closed-loop system
The feedback matrix \(\mathcal {G}\in \hbox {I}\!\hbox {R}^{m\times n}\) is given as \(\mathcal {G}=-R^{-1}\mathcal {B}^{\mathsf{T}}\mathcal {X}\), and \(\mathcal {X}\in \hbox {I}\!\hbox {R}^{n\times n}\) is the solution of the algebraic Riccati matrix equation
The algebraic Riccati equation has a unique positive definite solution \(\mathcal {X}\) if the pair \((\mathcal {A},\mathcal {B})\) is stabilizable and the pair \((\mathcal {C},\mathcal {A})\) is detectable (see Kwakernaak and Sivan 1972). Controllability of \((\mathcal {A},\mathcal {B})\) and observability of \((\mathcal {C},\mathcal {A})\) provide stronger conditions requiring
Under these conditions the closed-loop system (32) is globally asymptotically stable, i.e., all eigenvalues of the matrix \(\mathcal {A}-\mathcal {B}\mathcal {G}\) have a negative real part.
Assume that the linear system (30) is obtained from a non-linear system
by linearization around an equilibrium \(x_{\text {equi}}\) and around \(u\equiv 0\). The system of equations for computing \(x_{\text {equi}}\) is
Without restriction we can assume \(h(x_{\text {equi}})=0\) (otherwise we replace \(h(x)\) by \(h(x)-h(x_{\text {equi}})\)). Then, the matrices \(\mathcal {A},\, \mathcal {B}\), and \(\mathcal {C}\) are given by
Then, a result by Russel ((Russell 1979, Chapter IV)) implies that \(\hat{u}(t)=-\mathcal {G}\bigl (x(t)-x_{\text {equi}}\bigr )\) is a stabilizing control for the non-linear system (34), i.e., the equilibrium \(x(t)\equiv x_{\text {equi}}\) is asymptotically stable (but in general not globally asymptotically stable) for the nonlinear closed-loop system
Rights and permissions
About this article
Cite this article
de los Reyes, A.A., Jung, E. & Kappel, F. Stabilizing Control for a Pulsatile Cardiovascular Mathematical Model. Bull Math Biol 76, 1306–1332 (2014). https://doi.org/10.1007/s11538-014-9958-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-9958-2