Abstract
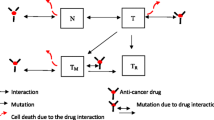
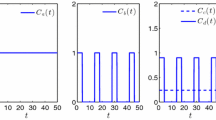
In this paper we develop a mathematical framework for describing multidrug resistance in cancer. To reflect the complexity of the underlying interplay between cancer cells and the therapeutic agent, we assume that the resistance level is a continuous parameter. Our model is written as a system of integro-differential equations that are parameterized by the resistance level. This model incorporates the cell density and mutation dependence. Analysis and simulations of the model demonstrate how the dynamics evolves to a selection of one or more traits corresponding to different levels of resistance. The emerging limit distribution with nonzero variance is the desirable modeling outcome as it represents tumor heterogeneity.















Similar content being viewed by others
References
Bellomo, N. (1997). Nonlinear models and problems in applied sciences from differential quadrature to generalized collocation methods. Math. Comput. Model., 26(4), 13–34.
Birkhead, B. G., Rakin, E. M., Gallivan, S., Dones, L., & Rubens, R. D. (1987). A mathematical model of the development of drug resistance to cancer chemotherapy. Eur. J. Cancer Clin. Oncol., 23(9), 1421–1427.
Bozic, I., Allen, B., & Nowak, M. A. (2012). Dynamics of targeted cancer therapy. Trends Mol. Med., 18(6), 311–316.
Brimacombe, K. R., Hall, M. D., Auld, D. S., Inglese, J., Austin, C. P., Gottesman, M. M., & Fung, K. L. (2009). A dual-fluorescence high-throughput cell line system for probing multidrug resistance. Assay Drug Dev. Technol., 7(3), 233–249.
Calsina, À., & Cuadrado, S. (2000). A model for the adaptive dynamics of the maturation age. Ecol. Model., 133, 33–43.
Calsina, À., & Cuadrado, S. (2004). Small mutation rate and evolutionary stable strategies in infinite dimensional adaptive dynamics. J. Math. Biol., 48, 135–159.
Champagnat, N., Ferrière, R., & Méléard, S. (2006). Unifying evolutionary dynamics: from individual stochastic processes to macroscopic models. Theor. Popul. Biol., 69(3), 297–321.
Coldman, A. J., & Goldie, J. H. (1985). Role of mathematical modeling in protocol formulation in cancer chemotherapy. Cancer Treat. Rep., 69(10), 1041–1048.
Coldman, A. J., & Goldie, J. H. (1986). A stochastic model for the origin and treatment of tumors containing drug-resistant cells. Bull. Math. Biol., 48(3–4), 279–292.
Desvillettes, L., Jabin, P. E., Mischler, S., & Raoul, G. (2008). On selection dynamics for continuous structured populations. Commun. Math. Sci., 6(3), 729–747.
Diekmann, O., Jabin, P. E., Mischler, S., & Perthame, B. (2005). The dynamics of adaptation: an illuminating example and a Hamilton–Jacobi approach. Theor. Popul. Biol., 67(4), 257–271.
Fister, K. R., & Panetta, J. C. (2003). Optimal control applied to competing chemotherapeutic cell-kill strategies. SIAM J. Appl. Math., 63(6), 1954–1971.
Fodal, V., Pierobon, M., Liotta, L., & Petricoin, E. (2011). Mechanisms of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer J., 17(2), 89–95.
Forys, U., & Marciniak-Czochra, A. (2003). Logistic equations in tumor growth modeling. Int. J. Appl. Math. Comput. Sci., 13(3), 317–325.
Gillet, J. P., & Gottesman, M. M. (2010). Mechanisms of multidrug reistance in cancer. Methods Mol. Biol., 596, 47–76.
Goldie, J. H., & Coldman, A. J. (1979). A mathematical model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat. Rep., 63(11–12), 1727–1733.
Goldie, J. H., & Coldman, A. J. (1983a). A model for resistance of tumor cells to cancer chemotherapeutic agents. Math. Biosci., 65, 291–307.
Goldie, J. H., & Coldman, A. J. (1983b). Quantative model for multiple levels of drug resistance in clinical tumors. Cancer Treat. Rep., 67(10), 923–931.
Goldie, J. H., & Coldman, A. J. (1998). Drug resistance in cancer: mechanisms and models. Cambridge: Cambridge University Press.
Goldie, J. H., Coldman, A. J., & Gudauskas, G. A. (1982). Rationale for the use of alternating non-cross resistant chemotherapy. Cancer Treat. Rep., 66(3), 439–449.
Grantab, R., Sivananthan, S., & Tannock, I. F. (2006). The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells. Cancer Res., 66(2), 1033–1039.
Hakanson, M., Kobel, S., Lutolf, M. P., Textor, M., Cukierman, E., & Charnley, M. (2012). Controlled breast cancer microarrays for the deconvolution of cellular multilayering and density effects upon drug responses. PLoS ONE, 7(6), e40141.
Harnevo, L. E., & Agur, Z. (1991). The dynamics of gene amplification described as a multitype compartmental model and as a branching process. Math. Biosci., 103(1), 115–138.
Jackson, T. L., & Byrne, H. (2000). A mathematical model to study the effects of drug resistance and vasculature on the response of solid tumors to chemotherapy. Math. Biosci., 164(1), 17–38.
Khain, E., & Sander, L. M. (2006). Dynamics and pattern formation in invasive tumor growth. Phys. Rev. Lett., 96(18), 188103.
Kimmel, M., & Axelrod, D. E. (1990). Mathematical models of gene amplification with applications to cellular drug resistance tumorigenicity. Genetics, 125(3), 633–644.
Komarova, N. (2006). Stochastic modeling of drug resistance in cancer. J. Theor. Biol., 239(3), 351–366.
Komarova, N., & Wodarz, D. (2005). Drug resistance in cancer: principles of emergence and prevention. Proc. Natl. Acad. Sci. USA, 102(27), 9714–9719.
Kreso, A., O’Brien, C. A., van Galen, P., Gal, O. I., Notta, F., et al. (2013). Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science, 339(6119), 543–548.
Laird, A. K. (1964). Dynamics of tumor growth. Br. J. Cancer, 18(3), 490–502.
Lavi, O., Gottesman, M. M., & Levy, D. (2012). The dynamics of drug resistance: a mathematical perspective. Drug Resist. Updat., 15(1–2), 90–97.
Lavi, O., Greene, J. M., Levy, D., & Gottesman, M. M. (2013). The role of cell density and intratumoral heterogeneity in multidrug resistance. Cancer Res., 73(24), 7168–7175.
Lavi, O., Greene, J. M., Levy, D., & Gottesman, M. M. Simplifying the complexity of resistance heterogeneity in metastasis, Trends in Molecular Medicine (2014, accepted).
Ledzewicz, U., & Schattler, H. (2006). Drug resistance in cancer chemotherapy as an optimal control problem. Discrete Contin. Dyn. Syst., 6(1), 129–150.
Long, H., Han, H., Yang, B., & Wang, Z. (2003). Opposite cell density-dependence between spontaneous and oxidative stress-induced apoptosis in mouse fibroblast L-cells. Cell. Physiol. Biochem., 13(6), 401–414.
Lorz, A., Mirrahimi, S., & Perthame, B. (2011). Dirac mass dynamics in multidimensional nonlocal parabolic equations. Commun. Partial. Differ. Equ., 36(6), 1071–1098.
Lorz, A., Lorenzi, T., Hochberg, M., Clairambault, J., & Perthame, B. (2013). Populational adaptive evolution, chemotherapeutic resistance and multiple anti-cancer therapies. Math. Model. Num. Anal., 47(2), 377–399.
Magal, P., & Webb, G. F. (2000). Mutation, selection and recombination in a model of phenotype evolution. Discrete Contin. Dyn. Syst., 6(1), 221–236.
Marusyk, A., & Cancer, K. P. (2013). Cancer cell phenotypes, in fifty shades of grey. Science, 339(6119), 528–529.
Michelson, S., & Slate, D. (1992). A mathematical model of the P-glycoprotein pump as a mediator of multidrug resistance. Bull. Math. Biol., 54(6), 1023–1038.
Nagane, M., Coufal, F., Lin, H., Bögler, O., Cavenee, W. K., & Huang, H. J. (1996). A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res., 56(21), 5079–5086.
Norton, L., & Simon, R. (1977a). Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat. Rep., 61(7), 1307–1317.
Norton, L., & Simon, R. (1977b). The growth curve of an experimental tumor following radiotherapy. J. Natl. Cancer Inst., 58(6), 1735–1741.
Norton, L., & Simon, R. (1986). The Norton–Simon hypothesis revisited. Cancer Treat. Rep., 70(1), 163–169.
Panetta, J. C. (1998). A mathematical model of drug resistance: heterogeneous tumors. Math. Biosci., 147(1), 41–61.
Perthame, B. (2007). Transport equations in biology, frontiers in mathematics. Basel: Birkhäuser.
Perthame, B., & Barles, G. (2008). Dirac concentrations in Lotka–Volterra parabolic PDEs. Indiana Univ. Math. J., 57(7), 3275–3301.
Qiao, L., & Farrell, G. C. (1999). The effects of cell density, attachment substratum and dexamethasone on spontaneous apoptosis of rate hepatocytes in primary culture. In Vitro Cell. Dev. Biol., Anim., 35(7), 417–424.
Saeki, K., Yuo, A., Kato, M., Miyazono, K., Yazaki, Y., & Takaku, F. (1997). Cell density-dependent apoptosis in HL-60 cells, which is mediated by an unknown soluble factor, is inhibited by transforming growth factor beta1 and overexpression of Bcl-2. J. Biol. Chem., 272(32), 20003–20010.
Saunders, N. A., Simpson, F., Thompson, E. W., Hill, M. M., Endo-Munoz, L., et al. (2012). Role of intratumoral heterogeneity in cancer drug resistance: molecular and clinical perspectives. EMBO J., 4(8), 675–684.
Schuster, R., & Schuster, H. (1995). Reconstruction models for the Ehrlich ascites tumor of the mouse. Math. Pop. Dyn., 2, 335–348.
Stein, A. M., Demuth, T., Mobley, D., Berens, M., & Sander, L. M. (2007). A mathematical model of glioblastoma tumor spheroid invasion in a three-dimensional in vitro experiment. Biophys. J., 921, 356–365.
Tomasetti, C., & Levy, D. (2010). An elementary approach to modeling drug resistance in cancer. Math. Biosci. Eng., 7(4), 905–918.
Weaver, V. M., Lelièvre, S., Lakins, J. N., Chrenek, M. A., Jones, J. C., Giancotti, F., Werb, Z., & Bissell, M. J. (2002). Beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell, 2(3), 205–216.
Zahir, N., & Weaver, V. M. (2004). Death in the third dimension: apoptosis regulation and tissue architecture. Curr. Opin. Genet. Dev., 14(1), 71–80.
Acknowledgements
We would like to thank George Leiman for editorial assistance. The work was supported by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute and was supported in part by a seed grant from the UMD-NCI Partnership for Cancer Technology. The work of D.L. was supported in part by the joint NSF/NIGMS program under Grant Number DMS-0758374 and by Grant Number R01CA130817 from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Appendix A
Appendix A
Proposition 1
Consider the integro-differential equation (6). If n(x,0)≥0 for all x∈[0,1], then
Proof
The global existence of continuously differentiable solutions of (1) can be obtained following standard arguments (see Perthame, 2007). For the positivity of n, since n≥0 at t=0, due to its continuity, if there exists a t ∗ for which n(x,t ∗)=0 for some x∈[0,1] (and n(x,t)>0 for t<t ∗), then n(y,t ∗)≥0 for all y. Hence, by (6),
which implies that n(x,t ∗) is nondecreasing at t ∗. Consequently, n(x,t) cannot pass through 0, as stated. □
Proof of Theorem 1
We follow the proof of Lorz et al. (2013), Lemma 2.2. Consider system (8). If r(x)−c(x)−d(x)<0 for all x∈[0,1], then due to the positivity of n(x,t), \(\frac{\partial n(x,t)}{\partial t} < 0\) for all x∈[0,1]. Hence, \(n(x,t) \xrightarrow[t \rightarrow \infty]{} 0\) in [0,1]. By Lebesgue’s Dominated Convergence Theorem, this implies that ρ(t)→0 as t→∞.
If, on the other hand, there exists x ∗ such that r(x ∗)−c(x ∗)−d(x ∗)=0, then these are fixed points of (8), and hence,
Now suppose that there exists x∈[0,1] such that r(x)−c(x)−d(x)>0. By the continuity of the growth parameters and the compactness of [0,1], r(x)−c(x)−d(x) achieves its maximum, say at \(\{x_{i} \}_{i=1}^{m}\). We note that it is possible that m=∞, or even that the set \(\{x_{i} \}_{i=1}^{m}\) is uncountable (in which case our notation should be altered).
To see that \(\rho(t) \xrightarrow[t \rightarrow \infty]{} \infty\), fix \(x_{j} \in \{x_{i} \}_{i=1}^{m}\) such that n(x j ,0)>0 (as the points where n=0 do not contribute to the growth). Then for all 0<λ<r(x j )−c(x j )−d(x j ), there exists γ λ such that
Let \(h_{\lambda}(t) := \int_{x_{j}-\gamma_{\lambda}}^{x_{j}+\gamma_{\lambda}} \! n(x,t) \, \mathrm{d} x\). Then
so that, for a positive constant h(0),
As \(\rho(t) = \int_{0}^{1} \! n(x,t) \, \mathrm{d} x \geq h_{\lambda}(t)\), (33) implies that \(\rho(t) \xrightarrow[t \rightarrow \infty]{} \infty\), as desired. To find the limiting distribution, note that for \(x \notin \{x_{i} \}_{i=1}^{m}\), choose λ such that
Then,
As \(\int_{0}^{1} \! \frac{n(x,t)}{\rho(t)} \, \mathrm{d} x = 1\), for all time t, we have the desired result, namely
with \(\sum_{i=1}^{m} a_{i} = 1\). If the number of maximizers is uncountable, a similar result will hold for a continuous measure. □
Proof of Theorem 2
Let n(x,t) satisfy (16). By Proposition 1, since n(x,t)≥0, ρ(t)≥0 as well. Let r M ,c m , and d m be constants such that r(x)≤r M , c(x)≥c m >0, and d(x)≥d m >0, and recall that G≥0. Hence, ρ′(t) can be bounded above by
Since \(G(\rho) \xrightarrow[\rho \rightarrow \infty]{} \infty\), there exists ρ M such that ρ(0)≤ρ M and r M −c m −G(ρ M )d m <0. This implies that at ρ=ρ M , ρ′(t)<0, and hence ρ(t)≤ρ M . □
Rights and permissions
About this article
Cite this article
Greene, J., Lavi, O., Gottesman, M.M. et al. The Impact of Cell Density and Mutations in a Model of Multidrug Resistance in Solid Tumors. Bull Math Biol 76, 627–653 (2014). https://doi.org/10.1007/s11538-014-9936-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-9936-8