Abstract
Salmonella is a major cause of bacterial foodborne disease. Human salmonellosis results in significant public health concerns and a considerable economic burden. Dairy cattle are recognized as a key source of several Salmonella serovars that are a threat to human health. To lower the risk of Salmonella infection, reduction of Salmonella prevalence in dairy cattle is important. Vaccination as a control measure has been applied for reduction of preharvest Salmonella prevalence on dairy farms. Salmonella vaccines are usually imperfect (i.e., vaccines may provide a partial protection for susceptible animals, reduce the infectiousness and shedding level, shorten the infectious period of infected animals, and/or curb the number of clinical cases), and evaluation of the potential impacts of imperfect Salmonella vaccines at the farm level is valuable to design effective intervention strategies. The objective of this study was to investigate the impact of imperfect Salmonella vaccines on the stochastic transmission dynamics in an adult dairy herd. To this end, we developed a semi-stochastic and individual-based continuous time Markov chain (CTMC) vaccination model with both direct and indirect transmission, and applied the CTMC vaccination model to Salmonella Cerro transmission in an adult dairy herd. Our results show that vaccines shortening the infectious period are most effective in reducing prevalence, and vaccines decreasing host susceptibility are most effective in reducing the outbreak size. Vaccines with multiple moderate efficacies may have the same effectiveness as vaccines with a single high efficacy in reducing prevalence, time to extinction, and outbreak size. Although the environment component has negligible contributions to the prevalence, time to extinction, and outbreak size for Salmonella Cerro in the herd, the relative importance of environment component was not assessed. This study indicates that an effective vaccination program against Salmonella Cerro spread in the herd can be designed with (1) vaccines with a single high efficacy in reducing either the infectious period or susceptibility of the host, or (2) if such single high efficacy vaccines are not available, vaccines with multiple moderate efficacies may be considered instead. These findings are also of general value for designing vaccination program for Salmonella serotypes in livestock.







Similar content being viewed by others
References
Allen, L. J. S. (2003). An introduction to stochastic processes with biology applications. New York: Prentice Hall.
Anderson, R. J., House, J. K., Smith, B. P., Kinde, H., Walker, R. L., Vande Steeg, B. J., & Breitmeyer, R. E. (2001). Epidemiologic and biological characteristics of salmonellosis in three dairy herds. J. Am. Vet. Med. Assoc., 219(3), 310–322.
Ball, F. G., & Lyne, O. D. (2002). Optimal vaccination polices for stochastic epidemics among a population of households. Math. Biosci., 177–178, 333–354.
Baumler, A. J. (2004). Foodborne Salmonella infections. In Preharvest and post-harvest food safety: contemporary issues and future directions. Oxford: Blackwell.
Chapagain, P. P., van Kessel, J. S., Karns, J. S., Wolfgang, D. R., Hovingh, E., Nelen, K. A., Schukken, Y. H., & Grohn, Y. T. (2008). A mathematical model of the dynamics of Salmonella Cerro infection in a US dairy herd. Epidemiol. Infect., 136(2), 263–272.
Clancy, D. (2005). A stochastic SIS infection model incorporating indirect transmission. J. Appl. Probab., 42, 726–737.
Cummings, K. J. (2010). The epidemiology and public health implications of salmonellosis in dairy cattle. Ph.D. thesis, Cornell University, Ithaca, NY.
Cummings, K. J., Warnick, L. D., Elton, M., Rodriguez-Rivera, L. D., Siler, J. D., Wright, E. M., Gröhn, Y. T., & Wiedmann, M. (2010). Salmonella enterica serotype Cerro among dairy cattle in New York: an emerging pathogen? Foodborne Pathog. Dis., 7(6), 659–665.
Dechet, A. M., Scallan, E., Gensheimer, K., Hoekstra, R., Gunderman-King, J., Lockett, J., Wrigley, D., Chege, W., & Sobel, J. (2006). Outbreak of multidrug-resistant Salmonella enterica serotype Typhimurium Definitive Type 104 infection linked to commercial ground beef, northeastern United States, 2003–2004. Clin. Infect. Dis., 42(6), 747–752.
Denagamage, T. N., O’Connor, A. M., Sargeant, J. M., Rajić, A., & McKean, J. D. (2007). Efficacy of vaccination to reduce Salmonella prevalence in live and slaughtered swine: a systematic review of literature from 1979 to 2007. Foodborne Pathog. Dis., 4(4), 539–549.
El-Gazzar, F. E., & Marth, E. H. (1992). Salmonellae, salmonellosis, and dairy foods: a review. J. Dairy Sci., 75(9), 2327–2343.
Gillespie, D. T. (2007). Stochastic simulation of chemical kinetics. Annu. Rev. Phys. Chem., 58, 35–55.
Gupta, A., Fontana, J., Crowe, C., Bolstorff, B., Stout, A., Van Duyne, S., Hoekstra, M. P., Whichard, J. M., Barrett, T. J., & Angulo, F. J. (2003). Emergence of multidrug-resistant Salmonella enterica serotype Newport infections resistant to expanded-spectrum cephalosporins in the United States. J. Infect. Dis., 188(11), 1707–1716.
Halloran, M. E., Struchiner, C. J., & Longini, I. M. Jr. (1999). Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am. J. Epidemiol., 146(10), 789–803.
Heider, L. C., Meiring, R. W., Hoet, A. E., Gebreyes, W. A., Funk, J. A., & Wittum, T. E. (2008). The effect of vaccination with a commercial subunit vaccine on the shedding of Salmonella enterica in non-clinically infected dairy cows. J. Am. Vet. Med. Assoc., 233(3), 466–469.
Helms, M., Vastrup, P., Gerner-Smidt, P., & Mølbak, K. (2002). Excess mortality associated with antimicrobial drug-resistant Salmonella typhimurium. Emerg. Infect. Dis., 8(5), 490–495.
Helton, J. C., & Davis, J. D. (2002). Illustration of sampling-based methods for uncertainty and sensitivity analysis. Risk Anal., 22(3), 591–622.
House, J. K., & Smith, B. P. (2004). Profitable strategies to control salmonellosis in dairy cattle. In Proceedings of the WBC Congress, Quebec, Canada.
House, J. K., Ontiveros, M. M., Blackmer, N. M., Dueger, E. L., Fitchhorn, J. B., McArthur, G. R., & Smith, B. P. (2001). Evaluation of an autogenous Salmonella bacterin and a modified live Salmonella serotype Choleraesuis vaccine on a commercial dairy farm. Am. J. Vet. Res., 62(12), 1897–1902.
Humphrey, T. (2004). Salmonella, stress responses and food safety. Nat. Rev. Microbiol., 2(6), 504–509.
Jones, T. F., Ingram, L. A., Cieslak, P. R., Vugia, D. J., Tobin-D’Angelo, M., Hurd, S., Medus, C., Cronquist, A., & Angulo, F. J. (2008). Salmonellosis outcomes differ substantially by serotype. J. Infect. Dis., 198(1), 109–114.
Keeling, M. J., & Rohani, R. (2008). Modeling of infectious diseases in humans and animals. Princeton: Princeton University Press.
Lanzas, C., Brien, S., Ivanek, R., Lo, Y., Chapagain, P. P., Ray, K. A., Ayscue, P., Warnick, L. D., & Gröhn, Y. T. (2008a). The effect of heterogeneous infectious period and contagiousness on the dynamics of Salmonella transmission in dairy cattle. Epidemiol. Infect., 136(11), 1496–1510.
Lanzas, C., Warnick, L. D., Ivanek, R., Ayscue, P., Nydam, D. V., & Gröhn, Y. T. (2008b). The risk and control of Salmonella outbreaks in calf-raising operations: a mathematical modeling approach. Vet. Res., 39(6), 61.
Lanzas, C., Warnick, L. D., James, K. L., Wright, E. M., Wiedmann, M., & Gröhn, Y. T. (2010). Transmission dynamics of a multidrug-resistant Salmonella Typhimurium outbreak in a dairy farm. Foodborne Pathog. Dis., 7(4), 467–474.
Lanzas, C., Lu, Z., & Gröhn, Y. T. (2011). Mathematical modeling of the transmission and control of foodborne pathogens and antimicrobial resistance at preharvest. Foodborne Pathog. Dis. doi:10.1089/fpd.2010.0643.
Leedom, J. M. (2006). Milk of nonhuman origin and infectious diseases in humans. Clin. Infect. Dis., 43(5), 610–615.
Lloyd, A. L. (2001). Realistic distributions of infectious periods in epidemic models: changing patterns of persistence and dynamics. Theor. Popul. Biol., 60(1), 59–71.
Lu, Z., Grohn, Y. T., Smith, R. L., Wolfgang, D. R., Van Kessel, J. A., & Schukken, Y. H. (2009). Assessing the potential impact of Salmonella vaccines in an endemically infected dairy herd. J. Theor. Biol., 259(4), 770–784.
Lurette, A., Belloc, C., Touzeau, S., Hoch, T., Ezanno, P., Seegers, H., & Fourichon, C. (2008). Modelling Salmonella spread within a farrow-to-finish pig herd. Vet. Res., 39(5), 49.
Lurette, A., Touzeau, S., Lamboni, M., & Monod, H. (2009). Sensitivity analysis to identify key parameters influencing Salmonella infection dynamics in a pig batch. J. Theor. Biol., 258(1), 43–52.
Majowicz, S. E., Musto, J., Scallan, E., Angulo, F. J., Kirk, M., O’Brien, S. J., Jones, T. F., Fazil, A., & Hoekstra, R. M. (2010). The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis., 50(6), 882–889.
Marino, S., Hogue, I. B., Ray, C. J., & Kirschner, D. E. (2008). A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol., 254(1), 178–196.
Martin, L. J., Fyfe, M., Doré, K., Buxton, J. A., Pollari, F., Henry, B., Middleton, D., Ahmed, R., Jamieson, F., Ciebin, B., McEwen, S. A., & Wilson, J. B. (2004). Increased burden of illness associated with antimicrobial-resistant Salmonella enterica serotype typhimurium infections. J. Infect. Dis., 189(3), 377–384.
Mead, P. S., Slutsker, L., Dietz, V., McCaig, L. F., Bresee, J. S., Shapiro, C., Griffin, P. M., & Tauxe, R. V. (1999). Food-related illness and death in the United States. Emerg. Infect. Dis., 5, 607–625.
Olsen, S. J., Ying, M., Davis, M. F., Deasy, M., Holland, B., Iampietro, L., Baysinger, C. M., Sassano, F., Polk, L. D., Gormley, B., Hung, M. J., Pilot, K., Orsini, M., Van Duyne, S., Rankin, S., Genese, C., Bresnitz, E. A., Smucker, J., Moll, M., & Sobel, J. (2004). Multidrug-resistant Salmonella Typhimurium infection from milk contaminated after pasteurization. Emerg. Infect. Dis., 10(5), 932–935.
Pradhan, A. K., Van Kessel, J. S., Karns, J. R., Wolfgang, D. R., Hovingh, E., Nelen, K. A., Smith, J. M., Whitlock, R. H., Fyock, T., Ladely, S., Fedorka-Cray, P. J., & Schukken, Y. H. (2009). Dynamics of endemic infectious diseases of animals and human importance on three dairy herds in the northeastern United States. J. Dairy Sci., 92, 1811–1825.
Smith, B. P., Habasha, F. G., Reina-Guierra, M., & Hardy, A. J. (1980). Immunization of calves against salmonellosis. Am. J. Vet. Res., 41(12), 1947–1951.
Steinbach, G., & Meyer, H. (1994). Efficacy of subcutaneous inoculation of calves with Murivac inactivated salmonellosis vaccine. Tierärztl. Prax., 22(6), 529–531.
Van Kampen, N. G. (1992). Stochastic processes in physics and chemistry. Netherlands: North Holland.
Van Kessel, J. S., Karns, J. S., Wolfgang, D. R., Hovingh, E., & Schukken, Y. H. (2007). Longitudinal study of a clonal, subclinical outbreak of Salmonella enterica subsp. enterica serovar Cerro in a U.S. dairy herd. Foodborne Pathog. Dis., 4(4), 449–461.
Varma, J. K., Molbak, K., Barrett, T. J., Beebe, J. L., Jones, T. F., Rabatsky-Ehr, T., Smith, K. E., Vugia, D. J., Chang, H. G., & Angulo, F. J. (2005). Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J. Infect. Dis., 191(4), 554–561.
Varma, J. K., Marcus, R., Stenzel, S. A., Hanna, S. S., Gettner, S., Anderson, B. J., Hayes, T., Shiferaw, B., Crume, T. L., Joyce, K., Fullerton, K. E., Voetsch, A. C., & Angulo, F. J. (2006). Highly resistant Salmonella Newport-MDRAmpC transmitted through the domestic US food supply: a FoodNet case-control study of sporadic Salmonella Newport infections, 2002–2003. J. Infect. Dis., 194(2), 222–230.
Voetsch, A. C., Van Gilder, T. J., Angulo, F. J., Farley, M. M., Shallow, S., Marcus, R., Cieslak, P. R., Deneen, V. C., & Tauxe, R. V. (2004). FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin. Infect. Dis., 38(Suppl. 3), S127–S134.
Weber, A., Bernt, C., Bauer, K., & Mayr, A. (1993). The control of bovine salmonellosis under field conditions using herd-specific vaccines. Tierarztl. Prax., 21(6), 511–516.
Xiao, Y., Bowers, R. G., Clancy, D., & French, N. P. (2005). Understanding the dynamics of Salmonella infections in dairy herds: a modelling approach. J. Theor. Biol., 233(2), 159–175.
Xiao, Y., Clancy, D., French, N. P., & Bowers, R. G. (2006). A semi-stochastic model for Salmonella infection in a multi-group herd. Math. Biosci., 200(2), 214–233.
Xiao, Y., Bowers, R. G., Clancy, D., & French, N. P. (2007). Dynamics of infection with multiple transmission mechanisms in unmanaged/managed animal populations. Theor. Popul. Biol., 71(4), 408–423.
Xu, Y., Allen, L. J., & Perelson, A. S. (2007). Stochastic model of an influenza epidemic with drug resistance. J. Theor. Biol., 248(1), 179–193.
You, Y., Rankin, S. C., Aceto, H. W., Benson, C. E., Toth, J. D., & Dou, Z. (2006). Survival of Salmonella enterica serovar Newport in manure and manure-amended soils. Appl. Environ. Microbiol., 72(9), 5777–5783.
Acknowledgements
We thank two anonymous reviewers for their constructive and valuable comments which greatly improve the content and presentation of our manuscript. This work was supported by USDA-ARS under the Regional Dairy Quality Management Alliance (RDQMA) Specific Cooperative Agreement.
Author information
Authors and Affiliations
Corresponding author
Appendix: The Semi-Stochastic Individual-Based Algorithm
Appendix: The Semi-Stochastic Individual-Based Algorithm
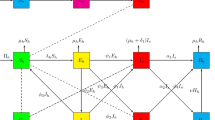
Gillespie algorithms are well known and have been applied to many problems where the master equation could not be solved analytically or numerically (Gillespie 2007). Here we described only the core part of the semi-stochastic individual-based algorithm, the time to next event for our imperfect Salmonella vaccination model. To find the time to the next event, we combined the direct and first reaction Gillespie algorithms (Keeling and Rohani 2008). The direct Gillespie algorithm was applied to all events listed in Table 2 except the recovery events. The time to the next event using the direct Gillespie algorithm, t d , is the solution of the following equation:
where the rand1 is a random number generated from a uniform distribution within 0–1, and the environment variable W(t) satisfies the first order ordinary differential equation of W(t) (Clancy 2005):
During the time interval (0,t d ), the number of animals in each compartment does not change; therefore, the analytical solution W(t) for the above equation can be found for t∈(0,t d ):
Substituting Eq. (4) into Eq. (2), the time to the next event t d was determined.
For the recovery events, the time since infection is important because the infectious period follows a gamma distribution. We applied the individual-based algorithm to consider this more realistic infectious period distribution in our simulation (Keeling and Rohani 2008). When an animal was infected as either unvaccinated (I) or vaccinated (Y), the time of this mth infectious animal recovering from I or Y (to R or Z), τ m , was predetermined by generating a random number following the gamma distribution, gamrnd(n,1/(nγ)) for I or gamrnd(n,(1−e γ )/(nγ)) for Y. We then used the first reaction Gillespie algorithm to find the time to the next event for all events:
where at the present time the number of all infectious animals is f≤N. If t next=t d , a second random number was generated to select which event was going to occur next, and then a third random number was used to find which animal was directly related to the next event. If t next=τ m , the status of the mth infectious animal was changed from I to R or Y to Z. Iterations including the above procedures ran until the prescribed time reached.
Rights and permissions
About this article
Cite this article
Lu, Z., Gröhn, Y.T., Smith, R.L. et al. Stochastic Modeling of Imperfect Salmonella Vaccines in an Adult Dairy Herd. Bull Math Biol 76, 541–565 (2014). https://doi.org/10.1007/s11538-013-9931-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-013-9931-5