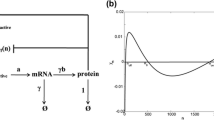
Abstract
Hysteresis, observed in many gene regulatory networks, has a pivotal impact on biological systems, which enhances the robustness of cell functions. In this paper, a general model is proposed to describe the hysteretic gene regulatory network by combining the hysteresis component and the transient dynamics. The Bouc–Wen hysteresis model is modified to describe the hysteresis component in the mammalian gene regulatory networks. Rigorous mathematical analysis on the dynamical properties of the model is presented to ensure the bounded-input-bounded-output (BIBO) stability and demonstrates that the original Bouc–Wen model can only generate a clockwise hysteresis loop while the modified model can describe both clockwise and counter clockwise hysteresis loops. Simulation studies have shown that the hysteresis loops from our model are consistent with the experimental observations in three mammalian gene regulatory networks and two E.coli gene regulatory networks, which demonstrate the ability and accuracy of the mathematical model to emulate natural gene expression behavior with hysteresis. A comparison study has also been conducted to show that this model fits the experiment data significantly better than previous ones in the literature. The successful modeling of the hysteresis in all the five hysteretic gene regulatory networks suggests that the new model has the potential to be a unified framework for modeling hysteresis in gene regulatory networks and provide better understanding of the general mechanism that drives the hysteretic function.










Similar content being viewed by others
References
Angeli, D., Ferrell, J. E. Jr., & Sontag, E. D. (2004). Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems. Proceedings of the National Academy of Sciences of the United States of America, 101(7), 1822–1827.
Atkinson, M. R., Savageau, M. A., Myers, J. T., & Ninfa, A. J. (2003). Development of genetic circuitry exhibiting toggle switch or oscillatory behavior in Escherichia coli. Cell, 113(5), 597–607.
Bagowski, C. P., & Ferrell, J. E. Jr. (2001). Bistability in the JNK cascade. Current Biology, 11, 1176–1182.
Becskei, A., Seraphin, B., & Serrano, L. (2001). Positive feedback in eukaryotic gene networks: cell differentiation by graded to binary response conversion. EMBO Journal, 20(10), 2528–2535.
Becskei, A., & Serrano, L. (2000). Engineering stability in gene networks by autoregulation. Nature, 405(6786), 590–593.
Buchler, E. N., Gerland, U., & Hwa, T. (2004). Nonlinear protein degradation and the function. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9559–9564.
Bouc, R. (1976). Forced vibrations of mechanical systems with hysteresis. In Proceedings of the fourth conference on nonlinear oscillations, p. 315.
Chang, D. E., Leung, S., Ninfa, J. A., et al. (2010). Building biological memory by linking positive feedback loops. Proceedings of the National Academy of Sciences of the United States of America, 107(1), 175–180.
Elowitz, B. M., & Leibler, S. (2000). A synthetic oscillatory network of transcriptional regulators. Nature, 403, 335–338.
Ferrell, J. E. Jr., Pomerening, J. R., Kim, S. Y., et al. (2009). Simple, realistic models of complex biological processes: positive feedback and bistability in a cell fate switch and a cell cycle oscillator. FEBS Letters, 583(24), 3999–4005.
Greber, D., & Fussenegger, M. (2007). Mammalian synthetic biology: engineering of sophisticated gene networks. Journal of Biotechnology, 130, 329–345.
Han, Z., Yang, L., MacLellan, R. W., Weiss, N. J., & Qu, Z. (2005). Hysteresis and cell cycle transitions: how crucial is it. Journal of Biophysics, 88, 1626–1634.
Ikhouane, F., & Rodellar, J. (2005). On the hysteretic Bouc–Wen model. Part I: Forced limit cycle characterization. Nonlinear Dynamics, 42(1), 63–78.
Justman, A. Q., Serber, Z., Ferrell, J. E. Jr., et al. (2009). Tuning the activation threshold of a kinase network by nested feedback loops. Science, 324, 509–512.
Kramer, B. P., Viretta, A. U., Daoud-El-Baba, M., Aubel, D., Weber, W., & Fussenegger, M. (2004). An engineered epigenetic transgene switch in mammalian cells. Nature Biotechnology, 22, 867–870.
Kramer, P.B., & Fussenegger, M. (2005). Hysteresis in a synthetic mammalian gene network. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9517–9522.
Longo, D., Hoffmann, A., Tsimring, L. S., & Hasty, J. (2010). Coherent activation of a synthetic mammalian gene network. Systems and Synthetic Biology, 4, 15–23.
Maeda, T. Y., & Sano, M. (2006). Regulatory dynamics of synthetic gene networks with positive feedback. Journal of Molecular Biology, 359, 1107–1124.
May, T., Eccleston, L., Wirth, D., et al. (2008). Bimodal and hysteretic expression in mammalian cells from a synthetic gene circuit. PLoS ONE, 3(6), e2372.
Nelder, J. A., & Mead, R. (1965). A simplex method for function minimization. Journal of Computing, 7, 308–313.
Ozbudak, M. E., Thattai, M., Lim, N.H., Shraiman, I.B., & Oudenaarden, V.A. (2004). Multistability in the lactose utilization network of Escherichia coli. Nature, 427, 737–740.
Pomerening, R. J., Sontag, D. E., & Ferrell, J. E. Jr. (2003). Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nature Cell Biology, 5(4), 346–351.
Portle, S., Iadevaia, S., San, Y. K., Bennett, N. G., & Mantzaris, N. (2009). Environmentally-modulated changes in fluorescence distribution in cells with oscillatory genetic network dynamics. Biotechnology Journal, 140, 203–217.
Qin, K. R., & Xiang, C. (2011). Hysteresis modeling for calcium-mediated ciliary beat frequency in airway epithelial cells. Mathematical Biosciences, 229(1), 101–108.
Sha, W., Moore, J., Chen, K., Lassaletta, A. D., Yi, C. S., Tyson, J. J., & Sible, J. C. (2003). Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proceedings of the National Academy of Sciences of the United States of America, 100(3), 975–980.
Solomon, J. M. (2003). Hysteresis meets the cell cycle. Proceedings of the National Academy of Sciences of the United States of America, 100(3), 771–772.
Stoer, J., & Bulirsch, R. (1993). Introduction to numerical analysis, 2nd edn. New York: Springer.
Tan, X., & Iyer, R. V. (2009). Modeling and control of hysteresis. IEEE Control Systems Magazine, 29, 26–28.
Tigges, M., Marquez-Lago, T. T., Stelling, J., & Fussenegger, M. (2009). A tunable synthetic mammalian oscillator. Nature Letters, 457, 309–312.
Toettcher, J. E., Mock, C., Batchelor, E., Loewer, A., & Lahav, G. (2010). A synthetic–natural hybrid oscillator in human cells. Proceedings of the National Academy of Sciences of the United States of America, 107(39), 17047–17052.
Weber, W., Kramer, B. P., & Fussenegger, M. (2007). A genetic time-delay circuitry in mammalian cells. Biotechnology and Bioengineering, 98, 894–902.
Wen, Y. K. (1976). Method of random vibration of hysteresis systems. ASCE Journal of Engineering Mechanics, 102, 249–263.
Xiong, W., & Ferrell, J. E. Jr. (2003). A positive-feedback-based bistable memory module that governs a cell fate decision. Nature, 426, 460–465.
Acknowledgements
The research reported here was supported by NUS Academic Research Fund R-263-000-483-112, the National Natural Science Foundation of China (Grant No. 11172060) and the Fundamental Research Funds for the Central Universities in China.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Model Validation on Another Mammalian Gene Network
Another synthetic mammalian positive feedback network was monitored in a lentiviral vector system with single-cell measurements (May et al. 2008). The enhanced green fluorescent protein (eGFP) mutant is regulated by the inducer doxycycline. The eGFP positive cell population is designated with HIGH and the eGFP negative cell population is designated with LOW. The experimental observed hysteresis loop between the percentage of the high cells and the concentration of the doxycycline is shown as the solid line in Fig. 10. A stochastic Markov process was presented based on the simplified reaction scheme as a mathematical description of the synthetic hysteretic gene regulatory circuit (May et al. 2008). The simulation result from the stochastic model shown as the dash-dot line in Fig. 10 predicted the existence of hysteresis which was verified by later experiment. However, the simulated hysteresis loop from the stochastic model did not match the experiment data very well. In this paper, we investigate whether our model can describe the hysteresis in this synthetic mammalian gene regulatory network more accurately. The concentration of doxycycline is the input (u(t)) and the percentage of the high cells is the output (y(t)). Due to the missing information on the time constant and threshold value in the experiment, the output value cannot be directly obtained from the general model. However, the experimental hysteresis loop is measured at steady state. When the time goes to infinity, the output value in Eq. (1) is constant for step input and its value is equal to the hysteresis component. Therefore, it is reasonable for us to obtain the steady state output value by deriving the value of the hysteresis component using the modified Bouc–Wen model in Eq. (3) to Eq. (8). The experiment data is extracted from Fig. 5B (May et al. 2008) and the whole parameter identification procedure is the same as that for Cell A and Cell B. The estimated parameter values are listed in Table 2 and the simulated loop from our model is shown as a dashed line in Fig. 10. Comparison results from the stochastic model and the new model shown in Fig. 10 demonstrate that our model shows substantially better agreement with the experiment result compared to the stochastic model though both models have captured the hysteretic feature in the gene regulatory network. Thus, it is evident to see the ability and accuracy of the new model to describe the hysteretic response in the mammalian gene regulatory network.
Appendix 2: Model Generalizability on E.coli Hysteretic Gene Regulatory Networks
In this section, two more examples of hysteretic gene networks in E.coli are utilized to test the generalizability of our model for modeling the hysteresis in gene regulatory network. Similarly, the steady state output value is obtained by deriving the hysteresis component value due to the missing information on the time constant and threshold value. The two sets of experiment data are extracted from Fig. 3A (Maeda and Sano 2006) and Fig. 3B (Chang et al. 2010) in the literature. The input values in both gene network are normalized as their order of magnitude vary sharply. The whole parameter identification procedure is the same as that for Cell A and Cell B. The estimated parameter values are in Table 2.
The chemical inducer IPTG is widely used as it cannot be metabolized by E.coli. A chromosomally expressing lactose repressor protein LacI, inhibits the expression of the gene coding for green fluorescence protein (GFP) by binding to the lactose operator. In the synthetic E.coli gene regulatory network, IPTG inactivates LacI to regulate [GFP] in a positive feedback loop. The interconnections between experiment measured [GFP] and [IPTG] shows hysteresis (Maeda and Sano 2006). By taking the normalized inducer concentration \(\operatorname{Log}([\mathit{IPTG}]\times10000)\) as the input (u(t)) and [GFP] as the output (y(t)), the new model is employed to characterize the hysteresis in the E.coli synthetic gene regulatory network. The simulated hysteresis loop shown in Fig. 11 demonstrates adequate agreement with the experiment data. This verifies the ability of the mathematical model to describe the hysteretic interaction between GFP and IPTG in the E.coli gene network.
IPTG induces the transcription of the gene coding for beta-galactosidase. The beta-galactosidase provides positive feedback that drives its own expression induced by IPTG, which inactivates repressor (Chang et al. 2010). By taking the normalized inducer concentration \(\operatorname{Log}([\mathit{IPTG}]\times100)\) as the input (u(t)) and the concentration of beta-galactoside as the output (y(t)), our general model is used to describe the hysteresis in E.coli synthetic gene regulatory network. Figure 12 shows the simulated hysteresis loop between beta-galactoside and the IPTG in this synthetic gene regulatory network. The strong consistence between the simulated result and the experiment data again demonstrates that the model can also describe the hysteresis in the synthetic gene regulatory network of E.coli.
Rights and permissions
About this article
Cite this article
Hu, J., Qin, K.R., Xiang, C. et al. Modeling of Hysteresis in Gene Regulatory Networks. Bull Math Biol 74, 1727–1753 (2012). https://doi.org/10.1007/s11538-012-9733-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-012-9733-1