Abstract
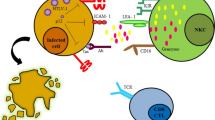
Human T-cell leukaemia/lymphoma virus type I (HTLV-I) is a retrovirus that has been identified as the causative agent of HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) and other illnesses. HTLV-I infects primarily CD4+ T cells and the transmission occurs through direct cell-to-cell contact. HAM/TSP patients harbor higher proviral loads in peripheral blood lymphocytes than asymptomatic carriers. Also, HAM/TSP patients exhibit a remarkably high number of circulating HTLV-I-specific CD8+ cytotoxic T lymphocytes (CTLs) in the peripheral blood. While CTLs have a protective role by killing the infected cells and lowering the proviral load, a high level of CTLs and their cytotoxicity are believed to be a main cause of the development of HAM/TSP. A mathematical model for HTLV-I infection of CD4+ T cells that incorporates the CD8+ cytotoxic T-cell (CTL) response is investigated. Our mathematical analysis reveals that the system can stabilize at a carrier steady-state with persistent viral infection but no CTL response, or at a HAM/TSP steady-state at which both the viral infection and CTL response are persistent. We also establish two threshold parameters R 0 and R 1, the basic reproduction numbers for viral persistence and for CTL response, respectively. We show that the parameter R 1 can be used to distinguish asymptomatic carriers from HAM/TSP patients, and as an important control parameter for preventing the development of HAM/TSP.
Similar content being viewed by others
References
Asquit, B., Bangham, C.R.M., 2007. Quantifying HTLV-I dynamics. Immunol. Cell Biol. 85, 280–286.
Bangham, C.M.R., 2000. The immune response to HTLV-I. Curr. Opin. Immunol. 12, 397–402.
Bangham, C.R., Meekings, K., Toulza, F., Nejmeddine, M., Majorovits, E., Asquith, B., Taylor, G.P., 2009. The immune control of HTLV-1 infection: selection forces and dynamics. Front. Biosci. 14, 2889–2903.
Cann, A.J., Chen, I.S.Y., 1996. Human T-cell leukemia virus type I and II. In: Fields, B.N., Knipe, D.M., Howley, P.M. (Eds.), Fields Virology, pp. 1849–1880. Lippincott-Raven Publishers.
Clark, D.R., De Boer, R.J., Wolthers, K.C., Miedema, F., 1999. T cell dynamics in HIV-1 infection. In: Dixon, F.J. (Ed.), Advances in Immunology, pp. 301–327. Academic Press, San Diego.
Elovaara, I., Koenig, S., Brewah, A.Y., Woods, R.M., Lehky, T., Jacobson, S., 1993. High human T cell lymphotropic virus type 1 (HTLV-1)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J. Exp. Med. 177, 1567–1573.
Gessain, A., Barin, F., Vernant, J.C., Gout, O., Maurs, L., Calender, A., de Thé, G., 1985. Antibodies to human T-lymphotropic virus type-I in patient with tropical spastic paraparesis. Lancet 2, 407–410.
Gómez-Acevedo, H., Li, M.Y., 2005. Backward bifurcation in a model for HTLV-I infection of CD4+ T cells. Bull. Math. Biol. 67, 101–114.
Greten, T.F., Slansky, J.E., Kubota, R., Soldan, S.S., Jaffee, E.M., Liest, T.P., Paradoll, D.M., Jacobson, S., Schneck, J.P., 1998. Direct visualization of antigen-specific T cells: HTLV-I Tax 11-19-specific CD8+ cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc. Natl. Acad. Sci. USA 95, 7568–7573.
Igakura, T., Stinchcombe, J.C., Goon, P.K.C., Taylor, G.P., Weber, J.N., Griffiths, G.M., Tanaka, Y., Osame, M., Bangham, C.R.M., 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleto. Science 299, 1713–1716.
Jacobson, S., 2002. Immunopathogenesis of HTLV-I associated neurological disease. J. Infect. Dis. 186, 187–192.
Kaplan, J.E., Osame, M., Kubota, H., et al., 1990. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J. Acquir. Immune Defic. Syndr. 3, 1096–1101.
Kira, J., Koyanagi, Y., Yamada, T., et al., 1991. Increased HTLV-I proviral DNA in HTLV-I associated myelopathy: a quantitative polymerase chain reaction study. Ann. Neurol. 29, 194–201.
Koenig, S., Woods, R.M., Brewah, Y.A., Newell, A.J., Jones, G.M., Boone, E., Adelsbergern, J.W., Baseler, M.W., Robinson, S.M., Jacobson, S., 1993. Characterization of MHC class I restricted cytotoxic T cell responses to tax in HTLV-1 infected patients with neurologic disease. J. Immunol. 151, 3874–3883.
Kubota, R., Fujiyoshi, T., Izumo, S., et al., 1993. Fluctuation of HTLV-I proviral DNA in peripheral blood mononuclear cells of HTLV-I-associated myelopathy. J. Neuroimmunol. 42, 147–154.
Kubota, R., Osame, M., Jacobson, S., 2000. Retrovirus: Human T-cell lymphotropic virus type I-associated diseases and immune dysfunction. In: Cunningham, M.W., Fujinami, R.S. (Eds.), Effects of Microbes on the Immune System, pp. 349–371. Lippincott Williams & Wilkins.
LaSalle, J.P., 1976. The Stability of Dynamical Systems. Regional Conference Series in Applied Mathematics. SIAM, Philadelphia.
Macchi, B., Faraoni, I., Zhang, J., Grelli, S., Favalli, C., Mastino, A., Bonmassar, E., 1997. AZT inhibits the transmission of human T-cell leukaemia/lymphoma virus type I to adult peripheral blood mononuclear cells in vitro. J. Gen. Virol. 78, 1007–1016.
Manns, A., Murphy, E.L., Wilk, R., Haynes, G., Figueroa, J.P., Hanchard, B., Barnett, M., Drummond, J., Waters, D., Cerney, M., Seals, J.R., Alexander, S.S., Lee, H., Blattner, W.A., 1991. Detection of early human T-cell lymphotropic virus type I antibody patterns during seroconversion among transfusion recipients. Blood 77, 896–905.
Mansky, L.M., 2000. In vivo analysis of human T-cell leukemia virus type 1 reverse transcription accuracy. J. Virol. 74, 9525–9531.
Mortreux, F., Kazanji, M., Gabet, A.-S., de Thoisy, B., Wattel, E., 2001. Two-step nature of human T-cell leukemia virus type 1 replication in experimentally infected squirrel monkeys (saimiri sciureus). J. Virol. 75, 1083–1089.
Mosley, A.J., Asquith, B., Bangham, C.R.M., 2005. Cell-mediated immune response to human T-lymphotropic virus type I. Viral Immun. 18, 293–305.
Nagai, M., Yamano, Y., Brennan, M.B., Mora, C.A., Jacobson, S., 2001. Increased HTLV-I proviral load and preferential expansion of HTLV-I Tax-specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann. Neurol. 50, 807–812.
Nelson, P.W., Murray, J.D., Perelson, A.S., 2000. A model of HIV-1 pathogenesis that includes an intracellular delay. Math. Biosci. 163, 201–215.
Nowak, M.A., Bangham, C.R.M., 1996. Population dynamics of immune responses to persistent viruses. Science 272, 74–79.
Nowak, M.A., May, R.M., 2000. Virus Dynamics: Mathematical Principles of Immunology and Virology. Oxford University Press, London.
Okochi, K., Sato, H., Hinuma, Y., 1984. A retrospective study on transmission of adult T-cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 46, 245–253.
Osame, M., Usuku, K., Izumo, S., Ijichi, N., Aminati, H., Igata, A., Matsumoto, M., Tara, M., 1986. HTLV-I-associated myelopathy: a new clinical entity. Lancet 1, 1031–1032.
Parker, C.E., Daenke, S., Nightingale, S., Bangham, C.R.M., 1992. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology 188, 628–636.
Perelson, A.S., 1989. Modeling the interaction of the immune system with HIV. In: Castillo-Chávez, C. (Ed.), Mathematical and Statistical Approaches to AIDS Epidemiology, Lecture Notes in Biomathematics, vol. 83, pp. 350–370. Springer, Berlin.
Perelson, A.S., Nelson, P.W., 1999. Mathematical analysis of HIV-I dynamics in vivo. SIAM Rev. 41, 3–44.
Richardson, J.H., Edwards, A.J., Cruickshank, J.K., Rudge, P., Dalgleish, A.G., 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64, 5682–5687.
Shiraki, H., Sagara, Y., Inoue, Y., 2003. Cell-to-cell transmission of HTLV-I. In: Sugamura, K., Uchiyam, T., Matsuoka, M., Kannagi, M. (Eds.), Two Decades of Adult T-cell Leukemia and HTLV-I Research, pp. 303–316. Japan Scientific Societies, Tokyo.
Wodarz, D., Nowak, M.A., Bangham, C.R.M., 1999. The dynamics of HTLV-I and the CTL response. Immunol. Today 20, 220–227.
Yamano, Y., Nagai, M., Brennan, M., Mora, C.A., Soldan, S.S., Tomaru, U., Takenouchi, N., Izumo, S., Osame, M., Jacobson, S., 2002. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8+ T cells and disease severity in HTLV-1-associated myelopathy (HAM/TSP). Blood 99, 88–94.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-Acevedo, H., Li, M.Y. & Jacobson, S. Multistability in a Model for CTL Response to HTLV-I Infection and Its Implications to HAM/TSP Development and Prevention. Bull. Math. Biol. 72, 681–696 (2010). https://doi.org/10.1007/s11538-009-9465-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-009-9465-z