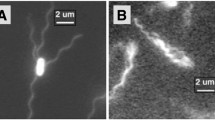
Abstract
We introduce a 3D model for a motile rod-shaped bacterial cell with a single polar flagellum which is based on the configuration of a monotrichous type of bacteria such as Pseudomonas aeruginosa. The structure of the model bacterial cell consists of a cylindrical body together with the flagellar forces produced by the rotation of a helical flagellum. The rod-shaped cell body is composed of a set of immersed boundary points and elastic links. The helical flagellum is assumed to be rigid and modeled as a set of discrete points along the helical flagellum and flagellar hook. A set of flagellar forces are applied along this helical curve as the flagellum rotates. An additional set of torque balance forces are applied on the cell body to induce counter-rotation of the body and provide torque balance. The three-dimensional Navier–Stokes equations for incompressible fluid are used to describe the fluid dynamics of the coupled fluid–microorganism system using Peskin’s immersed boundary method. A study of numerical convergence is presented along with simulations of a single swimming cell, the hydrodynamic interaction of two cells, and the interaction of a small cluster of cells.
Similar content being viewed by others
References
Arthurs, K.M., Moore, L.C., Peskin, C.S., Pitman, E.B., Layton, H.E., 1998. Modeling arteriolar flow and mass transport using the immersed boundary method. J. Comput. Phys. 147, 402–440.
Batchelor, G.K., 1970. Slender-body theory for particles of arbitrary cross section in Stokes flow. J. Fluid Mech. 44, 419–440.
Berg, H.C., 1975a. Bacterial behavior. Nature 254, 389–392.
Berg, H.C., 1975b. Chemotaxis in bacteria. Ann. Rev. 4, 119–136.
Berg, H.C., 1975c. How bacteria swim. Sci. Am. 233, 36–44.
Berg, H.C., 1986. Chemotaxis gene unveiled. Nature 321, 200–201.
Berg, H.C., 1993. Random Walks in Biology: Expanded Edition. Princeton University Press, Princeton.
Berg, H.C., 1996. Symmetries in bacterial motility. Proc. Natl. Acad. Sci. USA. 93, 14225–14228.
Berg, H.C., 2000. Motile behavior of bacteria. Physics Today on the Web-Cover Story. Available from: http://www.aip.org/pt/jcn00/berg.htm.
Berg, H.C., 2003. The rotary motor of bacterial flagella. Ann. Rev. Biochem. 72, 19–54.
Berg, H.C., Anderson, R.A., 1973. Bacteria swim by rotating their flagellar filaments. Nature 245, 380–382.
Berg, H.C., Brown, D.A., 1972. Chemotaxis in escherichia coli analyzed by three-dimensional tracking. Nature 239, 500–504.
Berg, H.C., Turner, L., 1990. Chemotaxis of bacteria in glass capillary array: Escherichia coli, motility, microchannel plate and light scattering. Biophys. J. 58, 919–930.
Berg, H.C., Turner, L., 1993. Torque generated by the flagellar motor of escherichia coli. Biophys. J. 65, 2201–2216.
Berg, H.C., Turner, L., 1994. Cells of escherichia coli swim either end forward. Cell Biol. 92, 477–479.
Berke, A.P., Turner, L., Berg, H.C., Lauga, E., 2008. Hydrodynamic attraction of swimming organisms by surfaces. Phys. Rev. Lett. 101, 038102.
Berry, R.M., Berg, H.C., 1999. Torque generated by the flagellar motor of escherichia coli while driven backward. Biophys. J. 76, 580–587.
Beyer, R.P. Jr., 1992. A computational model of the cochlea using the immersed boundary method. J. Comput Phys. 98(1), 145–162.
Bottino, D.C., 1998. Modeling viscoelastic networks and cell deformation in the context of the immersed boundary method. J. Comput. Phys. 147, 86–113.
Bottino, D.C., Fauci, L.J., 1998. A computational model of ameboid deformation and locomotion. Eur. Biophys. J. 27, 532–539.
Boyd, A., Simon, M., 1982. Bacterial chemotaxis. Ann. Rev. Physiol. 44, 501–517.
Brokaw, C.J., 1965. Non-sinusoidal bending wave of sperm falgella. J. Exp. Biol. 43, 155–169.
Brokaw, C.J., 2003. Swimming with three-dimensional flagellar bending waves. Available from: http://www.cco.caltech.edu/~brokawc/Suppl3D/Swim3D.pdf.
Brokaw, C.J., 2006. Falgella propulsion. J. Exp. Biol. Class. 209, 985–986.
Budrene, E.O., Berg, H.C., 1991. Complex pattern formed by motile cells of e. coli. Nature 349, 630–633.
Childress, S., 1981. Mechanics of Swimming and Flying. Cambridge University Press, Cambridge.
Cogan, N.G., Wolgemuth, C.W., 2005. Pattern formation by bacteria-driven flow. Biophys. J. 88, 2525–2529.
Cortez, R., Fauci, L.J., Cowen, N., Dillon, R., 2004. Simulation of swimming organisms: Coupling internal mechanics with external fluid dynamics. Comput. Sci. Eng. 6(3), 38–45.
Cox, R.G., 1970. The motion of long slender bodies in a viscous fluid. Part 1: General theory. J. Fluid Mech. 44, 791–810.
Darnton, N.C., Turner, L., Rojevsky, S., Berg, H.C., 2007. On torque and tumbling in swimming escherichia coli. J. Bacteriol. 189, 1756–1764.
Delden, C.V., Iglewski, B.H., 1998. Cell-to-cell signaling and pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4(4), 551–560.
DePamphilis, M.L., Adler, J., 1970. Fine structure and isolation of the Hook–Basal body complex of flagella from escherichia coli and bacillus subtilis. J. Bacteriol. 105(1), 384–395.
Dillon, R., Fauci, L.J., 2000a. An integrative model of internal axoneme mechanics and external fluid dynamics in ciliary beating. J. Theor. Biol. 207, 415–430.
Dillon, R., Fauci, L.J., 2000b. A Microscale Model of Bacterial and Biofilm Dynamics in Porous Media. Wiley, New York.
Dillon, R., Othmer, H.G., 1999. A mathematical model for outgrowth and spatial patterning of the vertebrate limb bud. J. Theor. Biol. 197, 295–330.
Dillon, R., Fauci, L.J., Fogelson, A.L., Gaver, D., 1988. Modeling biofilm processes using the immersed boundary method. J. Comput. Phys. 129, 57–73.
Dillon, R., Fauci, L.J., Gaver, D., 1995. A microscale model of bacterial swimming, chemotaxis and substrate transport. J. Theor. Biol. 177, 325–340.
Dillon, R., Fauci, L., Omoto, C., Yang, X.Z., 2007. Fluid dynamic models of flagellar and ciliary beating. NYAS 1101, 494–505.
Dillon, R., Painter, K., Owen, M., 2008. A single-cell-based model of multicellular growth using the immersed boundary method. AMS Contemp. Math. 466, 1–15.
DiLuzio, W.R., Turner, L., Mayer, M., Garstecki, P., Weibel, D.B., Berg, H., Whitesides, G.M., 2005. Escherichia coli swim on the right-hand side. Nature 435, 1271–1274.
Dombrowski, C., Cisneros, L., Chatkaew, S., Goldstein, R.E., Kessler, J.O., 2004. Self-concentration and large-scale coherence in bacterial dynamics. Phys. Rev. Lett. 93, 098103.
Doyle, T.B., Hawkins, A.C., McCarter, L.K., 2004. The complex flagellar torque generator of pseudomanas aeruginosa. J. Bacteriol. 186, 6341–6350.
Erban, R., Othmer, H.G., 2007. Taxis equations for amoeboid cells. J. Math. Biol. 54, 847–885.
Fauci, L.J., 1993. Computational modeling of the swimming of biflagellated algal cells. Contemp. Math. 141, 91–102.
Fauci, L.J., 1996. A computational model of the fluid dynamics of undulatory and flagellar swimming. Am. Zool. 36, 599–607.
Fauci, L.J., Fogelson, A.L., 1993. Truncated Newton method and the modeling of complex immersed elastic structures. J. Commun. Pure Appl. Math. XLVI, 787–818.
Fauci, L.J., McDonald, A., 1995. Sperm motility in the presence of boundaries. Bull. Math. Biol. 57(5), 679–699.
Fauci, L.J., Peskin, C.S., 1988. A computational model of aquatic animal locomotion. J. Comput. Phys. 77(1), 85–108.
Fogelson, A.L., 1984. A mathematical model and numerical method for study platelet adhesion and aggregation during blood clotting. J. Comput. Phys. 56(1), 111.
Fogelson, A.L., 1993. Continuum models of platelet aggregation: Mechanical properties and chemically-induced phase transitions. In: Fluid Dynamics in Biology, Contemporary Mathematics Series. American Mathematical Society, Providence.
Fund, D.D., Berg, H.C., 1995. Powering the flagellar motor of Escherichia coli with an external voltage source. Nature 375, 809–812.
Gabel, C.V., Berg, H.C., 2003. The speed of the flagellar rotary motor of Escherichia coli varies linearly with protonmotive force. PNAS 100, 8748–8751.
Gebremichael, Y., Ayton, G.S., Voth, G.A., 2006. Mesoscopic modeling of bacterial flagellar microhydrodynamics. Biophys. J. 91, 3640–3652.
Givelberg, E., Bunn, J., 2003. A comprehensive three-dimensional model of the cochlea. J. Comput. Phys. 191(2), 377–391.
Gracheva, M.E., Othmer, H.G., 2004. A continuum model of motility in ameboid cells. Bull. Math. Biol. 66, 167–193.
Gray, J., 1939a. Coornian lecture: Aspects of animal locomotion. Proc. R. Lond. Ser. B, Containing papers of a Biological Character 128, 28–62.
Gray, J., 1939b. Studies in animal locomotion: viii. The kinetics of locomotion of nereis diversicolor. J. Exp. Biol. 16, 9–17.
Gray, J., 1946. The mechanism of locomotion in snakes. J. Exp. Biol. 23, 101–120.
Gray, J., Hancock, G.J., 1955. The propulsion of sea-urchin spermatozoa. J. Exp. Biol. 32, 802–814.
Hancock, G.J., 1953. The self-propulsion of microscopic organisms through liquids. Proc. R. Soc. A 217, 96–121.
Harshey, R.M., 2003. Bacterial motility on a surface: many ways to a common goal. Ann. Rev. Microbiol 57, 249–273.
Higdon, J.J.L., 1979a. The generation of feeding currents by flagellar motions. J. Fluid Mech. 94, 305–330.
Higdon, J.J.L., 1979b. A hydrodynamics analysis of flagellar propulsion. J. Fluid Mech. 90, 685–711.
Higdon, J.J.L., 1979c. The hydrodynamics of flagellar propulsion: Helical waves. J. Fluid Mech. 94, 331–351.
Hopkins, M.M., 2002. Fauci, L.J., A computational model of the collective fluid dynamics of motile microorganisms.
Hsu, C.Y., 2007. A 3D Bacterial Swimming Model Coupled with External Fluid Mechanics Using the Immersed Boundary Method. Ph.D. Thesis.
Hsu, C.Y., Dillon, R., 2009. The hydrodynamic interaction of elastic structures with motile bacteria (in preparation).
Ishikawa, T., Hota, M., 2006. Interaction of two swimming paramecia. J. Exp. Biol. 209, 4452–4463.
Ishikawa, T., Pedley, T.J., 2007. Diffusion of swimming model microorganisms in a semi-dilute suspension. J Fluid Mech. 588, 437–462.
Ishikawa, T., Sekiya, G., Imai, Y., Yamaguchi, T., 2007. Hydrodynamic interactions between two swimming bacteria. Biophys. J. 93, 2217–2225.
Jánosi, I.M., Kessler, J.O., Horváth, V.K., 1998. Onset of bioconvection in suspensions of bacillus subtilis. Phys. Rev. E 58(4), 4793–4800.
Johnson, R.E., Brokaw, C.J., 1979. Flagellar hydrodynamics. Biophys. J. 25, 113–127.
Kudo, S., Imai, N., Nishitoba, M., Sugiyama, S., Magariyama, Y., 2005. Asymmetric swimming pattern of Vibrio alginolyticus cells with single polar flagella. FEMS Microbiol. Lett. 242, 221–225.
Larsen, S.H., Reader, R.W., Kort, E.N., Tso, W.W., Adler, J., 1974. Change in direction of flagellar rotation in the basis of the chemotactic response in Escherichia coli. Nature 249, 75–77.
Lauga, E., DiLuzio, W.R., Whitesides, G.M., Stone, H.A., 2006. Swimming in circles: Motion of bacteria near solid boundaries. Biophys. J. 90, 400–412.
Li, G., Tang, J.X., 2006. Low flagellar motor torque and high swimming efficiency of Caulobacter crescentus swarmer cells. Biophys. J. 91, 2726–2734.
Lighthill, J., 1975. Mathematical Biofluiddynamics. CBMS, vol. 17. SIAM, Philadelphia.
Lighthill, J., 1976. Flagellar hydrodynamics. SIAM Rev. 18(2), 161–229.
Lim, S., Peskin, C.S., 2004. Simulations of the whirling instability by the immersed boundary method. SIAM J. Sci. Comput. 25(6), 2066–2083.
Liu, Z., Papadopoulos, K.D., 1995. Unidirectional motility of Escherichia coli in restrictive capillaries. Appl. Environ. Microbiol. 61(10), 3567–3572.
Liu, Z., Chen, W., Papadopoulos, K.D., 1997. Bacterial motility, collisions and aggregation in a 3-μm-diameter capillary. Biotechnol. Bioeng. 53, 238–241.
Machin, K.E., 1958. Wave propagation along flagella. J. Exp. Biol. 35, 796–806.
Magariyama, Y., Masuda, S.Y., Takano, Y., Ohtani, T., Kudo, S., 2001. Difference between forward and backward swimming speeds of the single polar flagellated bacterium, Vibrio alginolyticus. FEMS Microbiol. Lett. 205, 343–347.
Magariyama, Y., Ichiba, M., Nakata, K., Baba, K., Ohtani, T., Kudo, S., Goto, T., 2005. Difference in bacterial motion between forward and backward swimming caused by the wall effect. Biophys. J. 88, 3648–3658.
Maki, N., Gestwicki, J.E., Lake, E.M., Kiesslingm, L.L., Adler, J., 2000. Motility and chemotaxis of filamentous cells of Escherichia coli. J. Bacteriol. 182(15), 4337–4342.
McCarter, L.L., 2001. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65, 445–462.
McQueen, D.M., Peskin, C.S., 2000. A three-dimensional computer model of the human heart for studying cardiac fluid dynamics. Comput. Graph. 34, 56–60.
McQueen, D.M., Peskin, C.S., 2001. Heart simulation by an immersed boundary method with formal second-order accuracy and reduced numerical viscosity. In: Aref, H., Phillips, J.W. (Eds.), Mechanics for a New Millennium, Proceedings of the International Conference on Theoretical and Applied Mechanics (ICTAM) 2000. Kluwer Academic, Dordrecht.
Mitchell, J.G., 2002. The energetics and scaling of search strategies in bacteria. Am. Nat. 160(6), 727–740.
Mitchell, J.G., Martinez-Alonso, M., Lalucat, J., Esteve, I., Brown, S., 1991. Velocity changes, long runs, and reversals in Chromatium minus swimming response. J. Bacteriol. 173, 997–1003.
Mittal, N., Budrene, E.O., Brenner, M.P., Oudenaarden, A.V., 2003. Motility of Escherichia coli cells in clusters formed by chemotactic aggregation. PNAS 100(3), 13259–13263.
Monaghan, J.J., 1994. Simulation free surface flows with SPH. J. Comput. Phys. 110, 399–406.
Nasseri, S., Phan-Thien, N., 1997. Hydrodynamic interaction between two nearby swimming micromachines. Comput. Mech. 20, 551–559.
Peskin, C.S., 1977. Numerical analysis of blood flow in the heart. J. Comput. Phys. 25(3), 221–249.
Peskin, C.S., 2002. The immersed boundary method. Acta Numer. 11, 1–39.
Peskin, C.S., McQueen, D.M., 1989. A three-dimensional computational model for blood flow in the heart: I. Immersed elastic fibers in a viscous incompressible fluid. J. Comput. Phys. 81, 372–405.
Peskin, C.S., McQueen, D.M., 1995. A general method for the computer simulation of biological systems interacting with fluids. In: Ellington, C.P., Pedley, T.J. (Eds.), Biological Fluid Dynamics. Company of Biologists, Cambridge.
Phan-Thien, N., Tran-Cong, T., Ramia, M., 1987. A boundary-element analysis of flagellar propulsion. J. Fluid Mech. 184, 533–549.
Prescott, L.M., Hartley, J.P., Klein, D.A., 1993. Microbiology, 2nd edn. Brown, Dufanque.
Ramia, M., Tullock, D.L., Phan-Thien, N., 1993. The role of hydrodynamic interaction in the locomotion of microorganisms. Biophys. J. 65, 755–778.
Reid, S.W., Leake, M.C., Chandler, J.H., Lo, C.J., Armitage, J.P., Berry, R.M., 2006. The maximum number of torque-generating units in the flagellar motor of Escherichia coli is at least 11. PNAS 103, 8066–8071.
Rejniak, K.A., 2007. An immersed boundary framework for modelling the growth of individual cells: an application to early tumour development. J. Theor Biol. 247, 186–204.
Rejniak, K.A., Dillon, R., 2007. A single-cell based model of the ductal tumor microarchitecture. Comput. Math. Meth. Med. 8, 51–69.
Rejniak, K.A., Kliman, H.J., Fauci, L.J., 2004. A computational model of the mechanics of growth of the villous trophoblast bilayer. Bull. Math. Biol. 66, 199–232.
Roberts, F.F. Jr., Doetsch, R.N., 1965. Some singular properties of bacterial flagella, with special reference to monotrichous forms. J. Bacteriol. 91(1), 414–421.
Roma, A.M., 1996. A multilevel self adaptive version of the immersed boundary method. Ph.D. Thesis, Department of Mathematics, New York University.
Rosar, M.E., Peskin, C.S., 2001. Fluid flow in collapsible elastic tubes: A three-dimensional numerical model. NY J. Math. 153, 509–534.
Savas, L., Duran, N., Savas, N., Önlen, Y., Ocak, S., 2005. The prevalence and resistance patterns of Pseudomonas aeruginosa in intensive care units in a university hospital. Turk J. Med. Sci. 35(5), 323–327.
Sowa, Y., Hotta, H., Homma, M., Ishijima, A., 2003. Torque-speed relationship of the Na+-driven flagellar motor of Vibrio alginolyticus. J. Mol. Biol. 327, 1043–1051.
Spormann, A.M., 1999. Gliding motility in bacteria: Insights from studies of Myxococcus xanthus. Microbiol. Mol. Biol. Rev. 63(3), 621–641.
Taylor, G.I., 1951a. The action of waving cylindrical tails in propelling microscopic organisms. Proc. R. Soc. A 211, 225–239.
Taylor, G.I., 1951b. Analysis of the swimming of microscopic organisms. Proc. R. Soc. A 209, 447–461.
Taylor, G.I., 1952. Analysis of the swimming of long and narrow animals. Proc. R. Soc. A 214(1117), 158–183.
Thar, R., Kühl, M., 2002. Conspicuous veils formed by vibrioid bacteria on sulfidic marine sediment. Appl. Environ. Microbiol. 68, 6310–6320.
Thar, R., Kühl, M., 2005. Complex pattern formation of marine gradient bacteria explained by a simple computer model. FEMS Microbiol. Lett. 246, 75–79.
Vesier, C.C., Yoganathan, A.P., 1992. A computer method for simulation of cardiovascular flow fields: Validation of approach. J. Comput. Phys. 99, 271–287.
Wang, N.T., Fogelson, A.L., 1999. Computational methods for continuum model of platelet aggregation. J. Comput. Phys. 151, 649–675.
Wang, Y., Hayat, T., Siddiqui, A.M., 2005. Gliding motion of bacteria on power-law slime. Math. Meth. Appl. Sci. 28, 329–347.
Wolgemuth, C.W., Charon, N.W., 2005. The kinky propulsion of spiroplasma. Cell 122(6), 827.
Wolgemuth, C.W., Powers, T.R., Goldstein, R.E., 2000. Twirling and whirling: Viscous dynamics of rotating elastic filaments. Phys. Rev. Lett. 84(7), 1623–1626.
Xing, J.H., Bai, F., Berry, R., Oster, G., 2006. Torque-speed relationship of the bacterial flagellar motor. PNAS 103, 1260–1265.
Yang, X.-Z., Dillon, R., Fauci, L., 2008. An integrative computational model of multiciliary beating. Bull. Math. Biol. 70, 1192–1215.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsu, CY., Dillon, R. A 3D Motile Rod-Shaped Monotrichous Bacterial Model. Bull. Math. Biol. 71, 1228–1263 (2009). https://doi.org/10.1007/s11538-009-9400-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-009-9400-3