Abstract
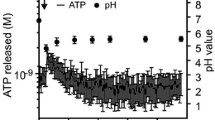
In goldfish hepatocytes, hypotonic exposure leads to cell swelling, followed by a compensatory shrinkage termed RVD. It has been previously shown that ATP is accumulated in the extracellular medium of swollen cells in a non-linear fashion, and that extracellular ATP (ATPe) is an essential intermediate to trigger RVD.
Thus, to understand how RVD proceeds in goldfish hepatocytes, we developed two mathematical models accounting for the experimental ATPe kinetics reported recently by Pafundo et al. in Am. J. Physiol. 294, R220–R233, 2008. Four different equations for ATPe fluxes were built to account for the release of ATP by lytic (J L ) and nonlytic mechanisms (J NL ), ATPe diffusion (J D ), and ATPe consumption by ectonucleotidases (J V ). Particular focus was given to J NL , defined as the product of a time function (J R ) and a positive feedback mechanism whereby ATPe amplifies J NL . Several J R functions (Constant, Step, Impulse, Gaussian, and Lognormal) were studied. Models were tested without (model 1) or with (model 2) diffusion of ATPe.
Mathematical analysis allowed us to get a general expression for each of the models. Subsequently, by using model dependent fit (simulations) as well as model analysis at infinite time, we observed that:
-
–
use of J D does not lead to improvements of the models.
-
–
Constant and Step time functions are only applicable when J R =0 (and thus, J NL =0), so that the only source of ATPe would be J L , a result incompatible with experimental data.
-
–
use of impulse, Gaussian, and lognormal J R s in the models led to reasonable good fits to experimental data, with the lognormal function in model 1 providing the best option.
Finally, the predictive nature of model 1 loaded with a lognormal J R was tested by simulating different putative in vivo scenarios where J V and J NL were varied over ample ranges.
Similar content being viewed by others
References
Akaike, H., 1992. Data analysis by statistical models. No To Hattatsu 24, 127–133.
Alleva, K.E., Espelt, M.E., Krumschnabel, G., Schwarzbaum, P.J., 2002. Identification of two distinct E-NTPDases in liver of goldfish (Carassius auratus L.). Comp. Biochem. Physiol. 131, 725–731.
Anderson, C.M., Bergher, J.P., Swanson, R.A., 2004. ATP-induced ATP release from astrocytes. J. Neurochem. 88, 246–256.
Burnstock, G., 2006. Purinergic signalling. Br. J. Pharmacol. 147, 172–181.
Burnstock, G., 2007. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797.
Chessell, I.P., Hatcher, J.P., Bountra, C., Michel, A.D., Hughes, J.P., Green, P., Egerton, J., Murfin, M., Richardson, J., Peck, W.L., Grahames, C.B., Casula, M.A., Yiangou, Y., Birch, R., Anand, P., Buell, G.N., 2005. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114, 386–396.
Dainty, J., 1963. The polar permeability of plant cell membranes to water. Protoplasma 1, 220–228.
Dainty, J., House, C.R., 1966. Unstirred layers in frog skin. J. Physiol. 182, 66–78.
Finkelstein, A., 1987. Water Movement through Lipid Bilayers, Pores, and Plasma Membranes. Wiley, New York.
Haussinger, D., 1996. The role of cellular hydration in the regulation of cell function. Biochem. J. 313, 697–710.
Hernández, J.A., Cristina, E., 1998. Modeling cell volume regulation in nonexcitable cells: the roles of the Na+ pump and of cotransport systems. Am. J. Physiol. 275, 1067–1080.
Hubley, M.J., Locke, B.R., Moerland, T.S., 1996. The effects of temperature, pH, and magnesium on the diffusion coefficient of ATP in solutions of physiological ionic strength. Biochim. Biophys. Acta 1291, 115–121.
Jakab, M., Fürst, J., Gschwentner, M., Bottà, G., Garavaglia, M.L., Bazzini, C., Rodighiero, S., Meyer, G., Eichmüller, S., Wöll, E., Chwatal, S., Ritter, M., Paulmichl, M., 2002. Mechanisms sensing and modulating signals arising from cell swelling. Cell. Physiol. Biochem. 12, 235–258.
Jans, D., Srinivas, S.P., Waelkens, E., Segal, A., Larivière, E., Simaels, J., Van Driessche, W., 2002. Hypotonic treatment evokes biphasic ATP release across the basolateral membrane of cultured renal epithelia (A6). J. Physiol. 545, 543–555.
Lazarowski, E.R., Boucher, R.C., Harden, T.K., 2003. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 64, 785–795.
Okada, Y., Maeno, E., Shimizu, T., Dezaki, K., Wang, J., Morishima, S., 2001. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol. 532, 3–16.
Pafundo, D.E., Mut, P., Pérez Recalde, M., González-Lebrero, R.M., Fachino, V., Krumschnabel, G., Schwarzbaum, P.J., 2004. Effects of extracellular nucleotides and their hydrolysis products on regulatory volume decrease of trout hepatocytes. Am. J. Physiol. 287, 833–843.
Pafundo, D.E., Chara, O., Faillace, M.P., Krumschnabel, G., Schwarzbaum, P.J., 2008. Kinetics of ATP release and cell volume regulation of hyposmotically challenged goldfish hepatocytes. Am. J. Physiol. 294, 220–233.
Pohl, P., Saparov, S.M., Antonenko, Y.N., 1998. The effect of a transmembrane osmotic flux on the ion concentration distribution in the immediate membrane vicinity measured by microelectrodes. Biophys. J. 72, 1711–1718.
Sabirov, R.Z., Okada, Y., 2005. ATP release via anion channels. Purinergic Signal. 1, 311–328.
Schulman, J.H., Teorell, T., 1938. On the boundary layer at membrane and monolayer interfaces. Trans. Faraday Soc. 34, 1337–1342.
Schwarzbaum, P.J., Frischmann, M.E., Krumschnabel, G., Rossi, R.C., Wieser, W., 1998. Functional role of ecto-ATPase activity in goldfish hepatocytes. Am. J. Physiol. 274, 1031–1038.
Suadicani, S.O., Brosnan, C.F., Scemes, E., 2006. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 26, 1378–1385.
Wang, Y., Roman, R., Lidofsky, S.D., Fitz, J.G., 1996. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc. Natl. Acad. Sci. 93, 12020–12025.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chara, O., Pafundo, D.E. & Schwarzbaum, P.J. Kinetics of Extracellular ATP from Goldfish Hepatocytes: A Lesson from Mathematical Modeling. Bull. Math. Biol. 71, 1025–1047 (2009). https://doi.org/10.1007/s11538-008-9392-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-008-9392-4