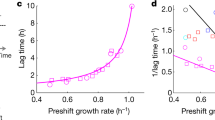
Abstract
During batch growth on mixtures of two growth-limiting substrates, microbes consume the substrates either sequentially (diauxie) or simultaneously. The ubiquity of these growth patterns suggests that they may be driven by a universal mechanism common to all microbial species. Recently, we showed that a minimal model accounting only for enzyme induction and dilution, the two processes that occur in all microbes, explains the phenotypes observed in batch cultures of various wild-type and mutant/recombinant cells (Narang and Pilyugin in J. Theor. Biol. 244:326–348, 2007). Here, we examine the extension of the minimal model to continuous cultures. We show that: (1) Several enzymatic trends, attributed entirely to cross-regulatory mechanisms, such as catabolite repression and inducer exclusion, can be quantitatively explained by enzyme dilution. (2) The bifurcation diagram of the minimal model for continuous cultures, which classifies the substrate consumption pattern at any given dilution rate and feed concentrations, provides a precise explanation for the empirically observed correlations between the growth patterns in batch and continuous cultures. (3) Numerical simulations of the model are in excellent agreement with the data. The model captures the variation of the steady state substrate concentrations, cell densities, and enzyme levels during the single- and mixed-substrate growth of bacteria and yeasts at various dilution rates and feed concentrations. This variation is well approximated by simple analytical expressions that furnish deep physical insights. (4) Since the minimal model describes the behavior of the cells in the absence of cross-regulatory mechanisms, it provides a rigorous framework for quantifying the effect of these mechanisms. We illustrate this by analyzing several data sets from the literature.
Similar content being viewed by others
References
Adhya, S., 2003. Suboperonic regulatory signals. Sci STKE 2003 (185), p. 22.
Bally, M., Wilberg, E., Kühni, M., Egli, T., 1994. Growth and regulation of enzyme synthesis in the nitrilotriacetic acid (NTA)-degrading bacterium Chelatobacter heintzii ATCC 29600. Microbiology 140, 1927–1936.
Barkley, M.D., Riggs, A.D., Jobe, A., Burgeois, S., 1975. Interaction of effecting ligands with lac repressor and repressor-operator complex. Biochemistry 14(8), 1700–1712.
Brinkmann, U., Babel, W., 1992. Simultaneous utilization of heterotrophic substrates by Hansenula polymorpha results in enhanced growth. Adv. Microbiol. Biotechnol. 37, 98–103.
Buettner, M.J., Spitz, E., Rickenberg, H.V., 1973. Cyclic adenosine 3’5’monophosphate in Escherichia coli. J. Bacteriol. 114(3), 1068–1073.
Buttin, G., 1963. Regulatory mechanisms in the biosynthesis of the enzymes of galactose metabolism in Escherichia coli k12. I the induced biosynthesis of galactokinase and the simultaneous induction of the enzymatic sequence. J. Mol. Biol. 7, 164–182.
Carroll, S.B., Grenier, J.K., Weatherbee, S.D., 2005. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design, 2nd edn., Blackwell, Oxford.
Chauvaux, S., 1996. CcpA and HPr(ser-P): mediators of catabolite repression in Bacillus subtilis. Res. Microbiol. 147(6–7), 518–522.
Clarke, P.H., Houldsworth, M.A., Lilly, M.D., 1968. Catabolite repression and the induction of amidase synthesis by Pseudomonas aeruginosa 8602 in continuous culture. J. Gen. Microbiol. 51, 225.
Collier, D.N., Hager, P.W., Phibbs, P.V., 1996. Catabolite repression control in the pseudomonads. Res. Microbiol. 147(6–7), 551–561.
Danchin, A., Dondon, L., Joseph, E., Ullmann, A., 1981. Transcription-translation coupling and polarity: A possible role of cyclic amp. Biochimie 63(5), 419–424.
Dean, A.C.R., 1972. Influence of environment on the control of enzyme synthesis. J. Appl. Chem. Biotechnol. 22, 245–259.
Dedem, G.V., Moo-Young, M., 1975. A model for diauxic growth. Biotechnol. Bioeng. 17, 1301–1312.
Egli, T., 1995. The ecological and physiological significance of the growth of heterotrophic microorganisms with mixtures of substrates. Adv. Microbiol. Ecol. 14, 305–386.
Egli, T., Harder, W., 1983. Growth of methylotrophs on mixed substrates. In: Crawford, R.L. (Ed.), Microbial Growth on C1 Compounds, pp. 330–337. ASM Press, Materials Park.
Egli, T., van Dijken, J.P., Veenhuis, J.P., Harder, W., Fiechter, A., 1980. Methanol metabolism in yeasts: Regulation of the synthesis of catabolic enzymes. Arch. Microbiol. 124, 115–121.
Egli, T., Käppeli, O., Fiechter, A., 1982a. Mixed substrate growth of methylotrophic yeasts in chemostat culture: Influence of dilution rate on the utilization of a mixture of methanol and glucose. Arch. Microbiol. 131, 8–13.
Egli, T., Käppeli, O., Fiechter, A., 1982b. Regulatory flexibility of methylotrophic yeasts in chemostat culture: Simultaneous assimilation of glucose and methanol at a fixed dilution rate. Arch. Microbiol. 131, 1–7.
Egli, T., Bosshard, C., Hamer, G., 1986. Simultaneous utilization of methanol-glucose mixtures by Hansenula polymorpha in chemostat: Influence of dilution rate and mixture composition on utilization pattern. Biotechnol. Bioeng. 28, 1735–1741.
Egli, T., Lendenmann, U., Snozzi, M., 1993. Kinetics of microbial growth with mixtures of carbon sources. Antonie van Leeuwenhoek 63, 289–298.
Epstein, W., Rothman-Denes, L.B., Hesse, J., 1975. Adenosine 3’5’cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 72(6), 2300–2304.
Eraso, P., Gancedo, J.M., 1984. Catabolite repression in yeasts is not associated with low levels of cAMP. Eur. J. Biochem. 141(1), 195–198.
Ferenci, T., 1996. Adaptation to life at micromolar nutrient levels: The regulation of Escherichia coli glucose transport by endoinduction and cAMP. FEMS Microbiol. Rev. 18(4), 301–317.
Fredrickson, A.G., 1976. Formulation of structured growth models. Biotechnol. Bioeng. 28, 1481–1486.
Guidi-Rontani, C., Danchin, A., Ullmann, A., 1984. Transcriptional control of polarity in Escherichia coli by cAMP. Mol. Gen. Genet. 195(1–2), 96–100.
Harder, W., Dijkhuizen, L., 1976. Mixed substrate utilization. In: Dean, A.C.R., Ellwood, D.C., Evans, C.G.T., Melling, J. (Eds.), Continuous Culture 6: Applications and New Fields, pp. 297–314. Ellis Horwood, Chichester, Chap. 23.
Harder, W., Dijkhuizen, L., 1982. Strategies of mixed substrate utilization in microorganisms. Phil. Trans. R. Soc. Lond. B 297, 459–480.
Hartner, F.S., Glieder, A., 2006. Regulation of methanol utilisation pathway genes in yeasts. Microb. Cell. Fact. 5, 39.
Herbert, D., Elsworth, R., Telling, R.C., 1956. The continuous culture of bacteria: A theoretical and experimental study. J. Gen. Microbiol. 14, 601–622.
Hogema, B.M., Arents, J.C., Bader, R., Eijkemans, K., Inada, T., Aiba, H., Postma, P.W., 1998. Inducer exclusion by glucose 6-phosphate in Escherichia coli. Mol. Microbiol. 28(4), 755–765.
Inada, T., Kimata, K., Aiba, H., 1996. Mechanism responsible for the glucose-lactose diauxie in Escherichia coli: Challenge to the cAMP model. Genes Cells 1, 293–301.
Jacob, F., Monod, J., 1961. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 3, 318–356.
Johnston, M., 1999. Feasting, fasting and fermenting. glucose sensing in yeast and other cells. Trends Genet. 15(1), 29–33.
Kimata, K., Takahashi, H., Inada, T., Postma, P., Aiba, H., 1997. cAMP receptor protein-cAMP plays a crucial role in glucose-lactose diauxie by activating the major glucose transporter gene in Escherichi coli. Proc. Natl. Acad. Sci. U.S.A. 94, 12914–12919.
Kovar, K., Chaloupka, V., Egli, T., 2002. A threshold concentration is required to initiate the degradation of 3-phenylpropionic acid in Escherichia coli. Acta Biotechnol. 22(3-4), 285–298.
Kovarova, K., Kach, A., Egli, T., Zehnder, A., 1997. Cultivation of Escherichia coli with mixtures of 3-phenylpropionic acid and glucose: Steady state growth kinetics. Appl. Environ. Microbiol. 63, 2619–2624.
Kovarova-Kovar, K., Egli, T., 1998. Growth kinetics of suspended microbial cells: From single-substrate-controlled growth to mixed-substrate kinetics. Microbiol. Mol. Biol. Rev. 62, 646–666.
Kovárová, K., Zehnder, A.J., Egli, T., 1996. Temperature-dependent growth kinetics of Escherichia coli ML 30 in glucose-limited continuous culture. J. Bacteriol. 178(15), 4530–4539.
Kremling, A., Bettenbrock, K., Laube, B., Jahreis, K., Lengeler, J.W., Gilles, E.D., 2001. The organization of metabolic reaction networks. III. Application for diauxic growth on glucose and lactose. Metab. Eng. 3(4), 362–379.
Kuo, J.-T., Chang, Y.-J., Tseng, C.-P., 2003. Growth rate regulation of lac operon expression in Escherichia coli is cyclic AMP dependent. FEBS Lett. 553(3), 397–402.
Lendenmann, U., 1994. Growth kinetics of Escherichia coli with mixtures of sugars. Ph.D. thesis, Swiss Federal Institute of Technology, Zürich, Switzerland.
Lendenmann, U., Snozzi, M., Egli, T., 1996. Kinetics of the simultaneous utilization of sugar mixtures by Escherichia coli in continuous culture. Appl. Environ. Microbiol. 62, 1493–1499.
Mach, H., Hecker, M., Mach, F., 1984. Evidence for the presence of cyclic adenosine monophosphate in Bacillus subtilis. FEMS Microbiol. 22, 27–30.
Mahadevan, R., Edwards, J.S., Doyle, F.J., 2002. Dynamic flux balance analysis of diauxic growth in Escherichia coli. Biophys. J. 83(3), 1331–1340.
Mandelstam, J., 1958. Turnover of protein in growing and non-growing populations of Escherichia coli. Biochem. J. 69(1), 110–119.
Matin, A., 1978. Microbial regulatory mechanisms at low nutrient concentrations as studied in a chemostat. In: Shilo, M. (Ed.), Strategies of Microbial Life in Extreme Environments, pp. 323–339. Verlag Chemie, Weinheim.
McGinnis, J.F., Paigen, K., 1969. Catabolite inhibition: a general phenomenon in the control of carbohydrate utilization. J. Bacteriol. 100(2), 902–913.
Monod, J., 1942. Recherches sur la croissance des cultures bactériennes [Studies on the growth of bacterial cultures]. Actual. Sci. Ind. 911, 1–215.
Morales, G., Linares, J.F., Beloso, A., Albar, J.P., Martínez, J.L., Rojo, F., 2004. The Pseudomonas putida Crc global regulator controls the expression of genes from several chromosomal catabolic pathways for aromatic compounds. J. Bacteriol. 186(5), 1337–1344.
Müller-Hill, B., 1996. The lac Operon, 1st edn., de Gruyter, Berlin.
Murray, J.D., 1989. Mathematical Biology. Biomathematics Texts, Springer, New York.
Narang, A., 1998a. The dynamical analogy between microbial growth on mixtures of substrates and population growth of competing species. Biotech. Bioeng. 59, 116–121.
Narang, A., 1998b. The steady states of microbial growth on mixtures of substitutable substrates in a chemostat. J. Theor. Biol. 190, 241–261.
Narang, A., 2006. Comparative analysis of some models of gene regulation in mixed-substrate microbial growth. J. Theor. Biol. 242(2), 489–501.
Narang, A., Pilyugin, S.S., 2007. Bacterial gene regulation in diauxic and nondiauxic growth. J. Theor. Biol. 244, 326–348.
Narang, A., Pilyugin, S.S., 2008. Bistability of the lac operon during growth of Escherichia coli on lactose and lactose + glucose. Bull. Math. Biol. 70(4), 1032–1064.
Narang, A., Konopka, A., Ramkrishna, D., 1997a. The dynamics of microbial growth on mixtures of substrates in batch reactors. J. Theor. Biol. 184, 301–317.
Narang, A., Konopka, A., Ramkrishna, D., 1997b. New patterns of mixed substrate growth in batch cultures of Escherichia coli K12. Biotech. Bioeng. 55, 747–757.
Neidhardt, F.C., Magasanik, B., 1956. The effect of glucose on the induced biosynthesis of bacterial enzymes in the presence and absence of inducing agents. Biochem. Biophys. Acta 21.
Notley-McRobb, L., Ferenci, T., 2000. Substrate specificity and signal transduction pathways in the glucose-specific enzyme II (EII(Glc)) component of the Escherichia coli phosphotransferase system. J. Bacteriol. 182(16), 4437–4442.
Oehler, S., Eismann, E.R., Krämer, H., Müller-Hill, B., 1990. The three operators of the lac operon cooperate in repression. EMBO J. 9(4), 973–979.
Overath, P., 1968. Control of basal level activity of β-galactosidase in Escherichia coli. Mol. Gen. Genet. 101(2), 155–165.
Perlman, R.L., Pastan, I., 1968. Regulation of β-galactosidase synthesis in Escherichia coli by cyclic adenosine 3’5’monophosphate. J. Biol. Chem. 243(20), 5420–5427.
Postma, P.W., Lengeler, J.W., Jacobson, G.R., 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57(3), 543–594.
Ptashne, M., Gann, A., 2002. Genes & Signals, Cold Spring Harbor Laboratory Press, Cold Spring Harbor.
Ramakrishna, R., Ramkrishna, D., Konopka, A., 1996. Cybernetic modeling of growth in mixed, substitutable substrate environments: Preferential and simultaneous utilization. Biotechnol. Bioeng. 52, 141–151.
Rudolph, J.M., Grady, C.P., 2001. Effect of media composition on yield values of bacteria growing on binary and ternary substrate mixtures in continuous culture. Biotechnol. Bioeng. 74(5), 396–405.
Rudolph, J.M., Grady, C.P.L., 2002. Catabolic enzyme levels in bacteria grown on binary and ternary substrate mixtures in continuous culture. Biotechnol. Bioeng. 79(2), 188–199.
Santillán, M., Mackey, M.C., 2004. Influence of catabolite repression and inducer exclusion on the bistable behavior of the lac operon. Biophys. J. 86(3), 1282–1292.
Savageau, M.A., 2001. Design principles for elementary gene circuits: Elements, methods, and examples. Chaos 11(1), 142–159.
Schleif, R., 2000. Regulation of the L-arabinose operon of Escherichia coli. Trends Genet. 16(12), 559–565.
Schmidt, S.K., Alexander, M., 1985. Effects of dissolved organic carbon and second substrates on the biodegradation of organic compounds at low concentrations. Appl. Environ. Microbiol. 49(4), 822–827.
Schmidt, S.K., Scow, K.M., Alexander, M., 1987. Kinetics of p-nitrophenol mineralization by a Pseudomonas sp.: Effects of second substrates. Appl. Environ. Microbiol. 53(11), 2617–2623.
Seeto, S., Notley-McRobb, L., Ferenci, T., 2004. The multifactorial influences of RpoS, Mlc and cAMP on ptsG expression under glucose-limited and anaerobic conditions. Res. Microbiol. 155(3), 211–215.
Shoemaker, J., Reeves, G.T., Gupta, S., Pilyugin, S.S., Egli, T., Narang, A., 2003. The dynamics of single-substrate continuous cultures: The role of transport enzymes. J. Theor. Biol. 222, 307–322.
Silver, R.S., Mateles, R.I., 1969. Control of mixed-substrate utilization in continuous cultures of Escherichia coli. J. Bacteriol. 97, 535–543.
Smith, S.S., Atkinson, D.E., 1980. The expression of β-galactosidase by Escherichia coli during continuous culture. Arch. Biochem. Biophys. 202(2), 573–581.
Toda, K., 1981. Induction and repression of enzymes in microbial cultures. J. Chem. Tech. Biotechnol. 31, 775–790.
Ullmann, A., 1974. Are cyclic AMP effects related to real physiological phenomena? Biochem. Biophys. Res. Commun. 57(2), 348–352.
Ullmann, A., Monod, J., 1968. Cyclic AMP as an antagonist of catabolite repression in Escherichia coli. FEBS Lett. 2(1), 57–60.
van Dijken, J.P., Weusthuis, R.A., Pronk, J.T., 1993. Kinetics of growth and sugar consumption in yeasts. Antonie Van Leeuwenhoek 63(3–4), 343–352.
Wanner, B.L., Kodaira, R., Neidhardt, F.C., 1978. Regulation of lac operon expression: Reappraisal of the theory of catabolite repression. J. Bacteriol. 136(3), 947–954.
Weickert, M.J., Adhya, S., 1993. The galactose regulon of Escherichia coli. Mol. Microbiol. 10(2), 245–251.
Winkler, H.H., Wilson, T.H., 1967. Inhibition of β-galactoside transport by substrates of the glucose transport system in Escherichia coli. Biochim. Biophys. Acta 135(5), 1030–1051.
Wong, P., Gladney, S., Keasling, J.D., 1997. Mathematical model of the lac operon: Inducer exclusion, catabolite repression, and diauxic growth on glucose and lactose. Biotechnol. Prog. 13(2), 132–143.
Wright, L.F., Milne, D.P., Knowles, C.J., 1979. The regulatory effects of growth rate and cyclic AMP levels on carbon catabolism and respiration in Escherichia coli K-12. Biochim. Biophys. Acta 583(1), 73–80.
Yagil, G., Yagil, E., 1971. On the relation between effector concentration and the rate of induced enzyme synthesis. Biophys. J. 11, 11–27.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noel, J.T., Narang, A. Gene Regulation in Continuous Cultures: A Unified Theory for Bacteria and Yeasts. Bull. Math. Biol. 71, 453–514 (2009). https://doi.org/10.1007/s11538-008-9369-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-008-9369-3