Abstract
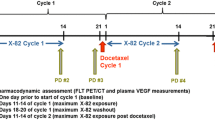
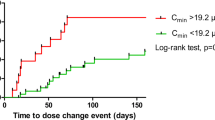
Pegylated liposomal doxorubicin (PLD) is active in breast, endometrial, and ovarian cancer. Preclinical data suggest that the combination of PLD with a mammalian target of rapamycin (mTOR) inhibitor has an additive effect. The safety and recommended phase two dose (RPTD) of temsirolimus in combination with PLD were assessed. 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT was performed for early response monitoring. Nineteen patients with advanced breast, endometrial, and ovarian cancer were treated with increasing doses of temsirolimus (10, 15, or 20 mg once weekly) and PLD (30 or 40 mg/m2 once every 4 weeks). PLD was initiated 2 weeks after start of temsirolimus. FDG-PET/CT was performed at baseline, after 2 and 6 weeks. Standardized uptake values (SUV), metabolic volume, and total lesion glycolysis (TLG, SUV × metabolic volume) were calculated. The RPTD was 15 mg temsirolimus and 40 mg/m2 PLD. Dose-limiting toxicities (DLT) were thrombocytopenia grade 3 with nose bleeding and skin toxicity grade 3. Most frequent treatment-related toxicities were nausea, fatigue, mucositis, and skin toxicity. Changes in TLG after 2 weeks predicted partial response (PR) after 10 weeks (p = 0.037). A rise in SUV between the second and sixth week predicted progression (PD) (p = 0.034) and was associated with worse progression free survival (PFS) (HR 1.068; p = 0.013). The RPTD was established at 15 mg temsirolimus weekly and PLD 40 mg/m2 once every 4 weeks and the combination was safe. Early response evaluation with FDG-PET/CT may predict subsequent radiological PR and PD. This trial is registered under number NCT0098263.



Similar content being viewed by others
References
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356(22):2271–2281. doi:10.1056/NEJMoa066838
Gridelli C, Maione P, Rossi A (2008) The potential role of mTOR inhibitors in non-small cell lung cancer. Oncologist 13(2):139–147. doi:10.1634/theoncologist.2007-0171
Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, Semiglazov V, Borner M, Salzberg M, Ostapenko V, Illiger HJ, Behringer D, Bardy-Bouxin N, Boni J, Kong S, Cincotta M, Moore L (2005) Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 23(23):5314–5322
Fleming G, Ma C, Huo D, Sattar H, Tretiakova M, Lin L, Hahn O, Olopade FO, Nanda R, Hoffman P, Naughton MJ, Pluard T, Conzen S, Ellis M (2012) Phase II trial of temsirolimus in patients with metastatic breast cancer. Breast Cancer Res Treat 136(2):355–363. doi:10.1007/s10549-011-1910-7
Behbakht K, Sill MW, Darcy KM, Rubin SC, Mannel RS, Waggoner S, Schilder RJ, Cai KQ, Godwin AK, Alpaugh RK (2011) Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol Oncol 123(1):19–26
Takano M, Kikuchi Y, Kudoh K, Goto T, Furuya K, Kikuchi R, Kita T, Fujiwara K, Shiozawa T, Aoki D (2011) Weekly administration of temsirolimus for heavily pretreated patients with clear cell carcinoma of the ovary: a report of six cases. Int J Clin Oncol 16(5):605–609. doi:10.1007/s10147-010-0177-z
Oza AM, Elit L, Tsao MS, Kamel-Reid S, Biagi J, Provencher DM, Gotlieb WH, Hoskins PJ, Ghatage P, Tonkin KS, Mackay HJ, Mazurka J, Sederias J, Ivy P, Dancey JE, Eisenhauer EA (2011) Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol 29(24):3278–3285
Piguet AC, Semela D, Keogh A, Wilkens L, Stroka D, Stoupis C, St-Pierre MV, Dufour JF (2008) Inhibition of mTOR in combination with doxorubicin in an experimental model of hepatocellular carcinoma. J Hepatol 49(1):78–87
Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M (2002) Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res 62(21):6141–6145
Mondesire WH, Jian W, Zhang H, Ensor J, Hung MC, Mills GB, Meric-Bernstam F (2004) Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res 10(20):7031–7042. doi:10.1158/1078-0432.CCR-04-0361
Tanaka M, Koul D, Davies MA, Liebert M, Steck PA, Grossman HB (2000) MMAC1/PTEN inhibits cell growth and induces chemosensitivity to doxorubicin in human bladder cancer cells. Oncogene 19(47):5406–5412
Jiang B-H, Liu L-Z (2008) Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat 11(3):63–76. doi:10.1016/j.drup.2008.03.001
Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ (2001) Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 19(14):3312–3322
Gordon AN, Tonda M, Sun S, Rackoff W (2004) Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol 95(1):1–8. doi:10.1016/j.ygyno.2004.07.011
Keller AM, Mennel RG, Georgoulias VA, Nabholtz J-M, Erazo A, Lluch A, Vogel CL, Kaufmann M, von Minckwitz G, Henderson IC, Mellars L, Alland L, Tendler C (2004) Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol 22(19):3893–3901. doi:10.1200/jco.2004.08.157
Muggia FM, Blessing JA, Sorosky J, Reid GC (2002) Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a gynecologic oncology group study. J Clin Oncol 20(9):2360–2364
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Desar IME, van Herpen CML, van Laarhoven HWM, Barentsz JO, Oyen WJG, van der Graaf WTA (2009) Beyond RECIST: molecular and functional imaging techniques for evaluation of response to targeted therapy. Cancer Treat Rev 35(4):309–321. doi:10.1016/j.ctrv.2008.12.001
Common Terminology Criteria for Adverse Events version 3.0. Available at: http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf.
Boellaard R, Oyen WJ, Hoekstra CJ, Hoekstra OS, Visser EP, Willemsen AT, Arends B, Verzijlbergen FJ, Zijlstra J, Paans AM, Comans EF, Pruim J (2008) The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging 35(12):2320–2333
Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J, Hoekstra OS, Pruim J, Marsden PK, Tatsch K, Hoekstra CJ, Visser EP, Arends B, Verzijlbergen FJ, Zijlstra JM, Comans EF, Lammertsma AA, Paans AM, Willemsen AT, Beyer T, Bockisch A, Schaefer-Prokop C, Delbeke D, Baum RP, Chiti A, Krause BJ (2010) FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging 37(1):181–200. doi:10.1007/s00259-009-1297-4
Picus J (2012) Phase I study of DOXIL and temsirolimus in resistant solid mailgnancies. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00703170.
Thornton KA, Chen AR, Trucco MM, Shah P, Wilky BA, Gul N, Carrera-Haro MA, Ferreira MF, Shafique U, Powell JD, Meyer CF, Loeb DM (2013) A dose-finding study of temsirolimus and liposomal doxorubicin for patients with recurrent and refractory bone and soft tissue sarcoma. Int J Cancer. doi:10.1002/ijc.28083
Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, Pujade-Lauraine E, Park YC, Parekh TV, Poveda AM (2012) Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: overall survival analysis. Eur J Cancer 48(15):2361–2368
Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, Volgger B, Vergote I, Pignata S, Ferrero A, Sehouli J, Lortholary A, Kristensen G, Jackisch C, Joly F, Brown C, Le Fur N, du Bois A (2010) Pegylated liposomal Doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol 28(20):3323–3329
Steffensen KD, Waldstrom M, Pallisgard N, Lund B, Bergfeldt K, Wihl J, Keldsen N, Marth C, Vergote I, Jakobsen A (2012) Panitumumab and pegylated liposomal doxorubicin in platinum-resistant epithelial ovarian cancer with KRAS wild-type: the PaLiDo Study, a phase II nonrandomized multicenter study. Int J Gynecol Cancer 3:3
Trafalis DT, Alifieris C, Krikelis D, Tzogkas N, Stathopoulos GP, Athanassiou AE, Sitaras NM (2012) Topotecan and pegylated liposomal doxorubicin combination as palliative treatment in patients with pretreated advanced malignant pleural mesothelioma. Int J Clin Pharmacol Ther 50(7):490–499
Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, Del Medico P, Scaltriti L, Katsaros D, Priolo D, Scambia G (2008) Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol 26(6):890–896
Kim RJ, Peterson G, Kulp B, Zanotti KM, Markman M (2005) Skin toxicity associated with pegylated liposomal doxorubicin (40 mg/m2) in the treatment of gynecologic cancers. Gynecol Oncol 97(2):374–378. doi:10.1016/j.ygyno.2004.12.057
Markman M, Kennedy A, Webster K, Peterson G, Kulp B, Belinson J (2000) Phase 2 Trial of liposomal doxorubicin (40 mg/m2) in platinum/paclitaxel-refractory ovarian and fallopian tube cancers and primary carcinoma of the peritoneum. Gynecol Oncol 78(3):369–372. doi:10.1006/gyno.2000.5921
Weber WA, Figlin R (2007) Monitoring cancer treatment with PET/CT: does it make a difference? J Nucl Med 48(Suppl 1):36S–44S
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, Casilla C, Fazzari M, Srivastava N, Yeung HW, Humm JL, Guillem J, Downey R, Karpeh M, Cohen AE, Ginsberg R (1999) Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 2(3):159–171
Funding
This work was supported by the manufacturers of temsirolimus and PLD: Wyeth Pharmaceuticals, Pfizer bv, Schering Plough, and Jansen-Cilag. These sponsors had no involvement in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Conflict of interest
This work was supported by the manufacturers of temsirolimus and PLD: Wyeth Pharmaceuticals, Pfizer bv, Schering Plough, and Jansen-Cilag to CvH and NO. WdG has conducted preclinical studies supported by Pfizer bv and Wyeth pharmaceuticals, which also supported this study. All remaining authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Petronella B. Ottevanger and Carla M. L. van Herpen contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Boers-Sonderen, M.J., de Geus-Oei, LF., Desar, I.M.E. et al. Temsirolimus and pegylated liposomal doxorubicin (PLD) combination therapy in breast, endometrial, and ovarian cancer: phase Ib results and prediction of clinical outcome with FDG-PET/CT. Targ Oncol 9, 339–347 (2014). https://doi.org/10.1007/s11523-014-0309-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-014-0309-x