Abstract
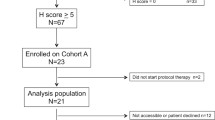
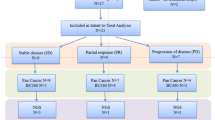
Preclinical models suggested that activating mutations of the PIK3CA gene are associated with sensitivity to inhibitors of the mammalian target of rapamycin (mTOR). In breast cancers, PIK3CA mutations are associated with estrogen receptor (ER) positivity. We therefore performed an open-label single arm phase II study of the rapamycin analog, temsirolimus, at a dose of 25 mg weekly, in women with pretreated breast cancers that were positive for ER, PR, or HER2. Archived formalin-fixed paraffin embedded tumor was collected for immunohistochemical evaluation of components of the PI3K/Akt/mTOR pathway and PIK3CA mutation analysis. Thirty-one patients were enrolled. There were no major objective responses; however, three patients had stable disease for over 24 weeks. Twenty-three tumor samples were available for mutational analysis. There were five tumors with PIK3CA mutations; no association was found between prolonged stable disease and PIK3CA mutation or any immunohistochemical marker. There was a trend toward improved progression free survival (PFS) for patients with positive nuclear staining for phospho-Akt308. One patient remains on study four and a half years after starting therapy; her tumor did not have a PIK3CA mutation. We conclude that single agent temsirolimus has minimal activity in a population of women with heavily pretreated breast cancer. We found no evidence that either absence of immunohistochemical staining for PTEN or mutations in the hotspot domains of PIK3CA in the primary tumor were associated with clinical benefit.
Similar content being viewed by others
Abbreviations
- PIK3CA :
-
Gene encoding phosphoinositide-3-kinase, catalytic subunit
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
Mammalian target of rapamycin complex 1
- mTORC2:
-
Mammalian target of rapamycin complex 2
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- ECOG:
-
Eastern Cooperative Oncology Group
- ANC:
-
Absolute neutrophil count
- CYP3A4:
-
Cytochrome P450 3A4
- RECIST:
-
Response evaluation criteria in solid tumors
- FFPE:
-
Formalin-fixed paraffin embedded
- PTEN:
-
Phosphatase and tensin homolog
- SGK:
-
Serum and glucocorticoid-induced protein kinase
- PDK:
-
Pyruvate dehydrogenase kinase
- TBS:
-
Tris-buffered saline
- H&E:
-
Hematoxylin and eosin
- PCR:
-
Polymerase chain reaction
- CR:
-
Complete response
- PR:
-
Partial response
- IHC:
-
Immunohistochemistry
- PFS:
-
Progression free survival
- IGF-1R:
-
Insulin-like growth factor receptor
- FISH:
-
Fluorescent in situ hybridization
- Ig:
-
Immunoglobulin
- NA:
-
Not available
References
Yu K, Toral-Barza L, Discafani C, Zhang WG, Skotnicki J, Frost P et al (2001) mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer 8(3):249–258
Kang S, Bader AG, Vogt PK (2005) Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA 102(3):802–807
Kalinsky K, Jacks LM, Heguy A, Patil S, Drobnjak M, Bhanot UK et al (2009) PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res 15(16):5049–5059
Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X et al (2005) PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 65(7):2554–2559
Hernandez-Aya LF, Gonzalez-Angulo AM (2011) Targeting the phosphatidylinositol 3-kinase signaling pathway in breast cancer. Oncologist 16(4):404–414
Ihle NT, Lemos R Jr, Wipf P, Yacoub A, Mitchell C, Siwak D et al (2009) Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res 69(1):143–150
Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C et al (2005) Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 23(23):5314–5322
Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J et al (2004) Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res 10(3):1013–1023
Ellard SL, Clemons M, Gelmon KA, Norris B, Kennecke H, Chia S et al (2009) Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol 27(27):4536–4541
Barbareschi M, Buttitta F, Felicioni L, Cotrupi S, Barassi F, Del Grammastro M et al (2007) Different prognostic roles of mutations in the helical and kinase domains of the PIK3CA gene in breast carcinomas. Clin Cancer Res 13(20):6064–6069
Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J et al (2009) Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27(16):2630–2637
Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS et al (2011) PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Res 10(3):558–565
Oza AM, Elit L, Tsao M-S, Kamel-Reid S, Biagi J, Provencher DM et al (2011) Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol 29(24):3278–3285
Dupont Jensen J, Laenkholm AV, Knoop A, Ewertz M, Bandaru R, Liu W et al (2011) PIK3CA mutations may be discordant between primary and corresponding metastatic disease in breast cancer. Clin Cancer Res 17(4):667–677
Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, Sahin A, Liu S, Barrera JA et al (2011) PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther 10(6):1093–1101
Ellis MJ, Lin L, Crowder R, Tao Y, Hoog J, Snider J et al (2010) Phosphatidyl-inositol-3-kinase alpha catalytic subunit mutation and response to neoadjuvant endocrine therapy for estrogen receptor positive breast cancer. Breast Cancer Res Treat 119(2):379–390
Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R et al (2001) Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA 98(18):10314–10319
Shi Y, Gera J, Hu L, Hsu JH, Bookstein R, Li W et al (2002) Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res 62(17):5027–5034
Steelman LS, Navolanic PM, Sokolosky ML, Taylor JR, Lehmann BD, Chappell WH et al (2008) Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitors. Oncogene 27(29):4086–4095
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M et al (2008) An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 68(15):6084–6091
Dan S, Okamura M, Seki M, Yamazaki K, Sugita H, Okui M et al (2010) Correlating phosphatidylinositol 3-kinase inhibitor efficacy with signaling pathway status: in silico and biological evaluations. Cancer Res 70(12):4982–4994
Di Nicolantonio F, Arena S, Tabernero J, Grosso S, Molinari F, Macarulla T et al (2010) Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest 120(8):2858–2866
Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H et al (2009) Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci USA 106(52):22299–22304
Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M et al (2008) NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res 68(19):8022–8030
Weigelt B, Warne PH, Downward J (2011) PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitory drugs. Oncogene 30(29):3222–3233
O’Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, Punnoose EA et al (2010) Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res 16(14):3670–3683
Marty B, Maire V, Gravier E, Rigaill G, Vincent-Salomon A, Kappler M et al (2008) Frequent PTEN genomic alterations and activated phosphatidylinositol 3-kinase pathway in basal-like breast cancer cells. Breast Cancer Res 10(6):R101
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H et al (2005) Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65(16):7052–7058
Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA et al (2004) PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6(2):117–127
Clark AS, West K, Streicher S, Dennis PA (2002) Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther 1(9):707–717
Bachelot T, Bourgier C, Cropet C, Guastalla JP, Ferrero JM, Leger-Falandry C et al (2010) TAMRAD: a GINECO randomized phase II trial of everolimus in combination with tamoxifen versus tamoxifen alone in patients (pts) with hormone-receptor positive, HER2 negative metastatic breast cancer (MBC) with prior exposure to aromatase inhibitors (AI). San Antonio Breast Conference
Bhattacharyya GS, Biswas J, Singh JK, Singh M, Govindbabu K, Ranade AA et al (2011) Breast cancer-early and advanced disease: breast cancer-advanced disease. European Multidisciplinary Cancer Congress
Dalenc F, Campone M, Hupperets P, O’Regan R, Manlius C, Vittori L et al (2010) Everolimus in combinatoin with weekly paclitaxel and trastuzumab in patients (pts) with HER2-overexpressing metastatic breast cancer (MBC) with prior resistance to trastuzumab and taxanes: A multcenter phase II clinical trial. J Clin Oncol 28(15S):117s
Morrow PH, Wulf GM, Booser DJ, Moore JA, Flores PR, Krop IE et al (2010) Phase I/II trial of everolimus (RAD001) and trastuzumab in patients with trastuzumab-resistant, HER2-overexpressing breast cancer. J Clin Oncol 28S(15):117s
Chow LWC, Sun Y, Jassem J, Baselga J, Hayes DF, Wolff AC et al (2006) Phase 3 study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancer. Breast Cancer Res Treat 100(1):s286
Acknowledgments
This research was supported by the National Cancer Institute (P30CA1459931 S1 and P30CA91842 S2) and the Avon Foundation, as well as the University of Chicago Breast Cancer SPORE (P50 CA125183).
Conflict of interest
The authors declare that they have no relevant conflicts of interest except for Dr Fleming, who in the past received partial support (including drug) for costs of an unrelated investigator-initiated trial using Temsirolimus from Wyeth. The Temsirolimus for the trial reported in this manuscript was supplied by the NCI, and there was no financial support from Wyeth.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fleming, G.F., Ma, C.X., Huo, D. et al. Phase II trial of temsirolimus in patients with metastatic breast cancer. Breast Cancer Res Treat 136, 355–363 (2012). https://doi.org/10.1007/s10549-011-1910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1910-7