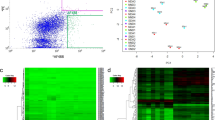
Abstract
Maternal smoking during pregnancy is associated with low birth weight, increased risk of stillbirth, conduct disorder, attention-deficit/hyperactivity disorder and neurocognitive deficits. Ventral tegmental area dopamine (DA) neurons in the mesocorticolimbic pathway were suggested to play a critical role in these pathological mechanisms induced by nicotine. Nicotine-mediated changes in genetic expression during pregnancy are of great interest for current researchers. We used patch clamp methods to identify and harvest DA and non-DA neurons separately and assayed them using oligonucleotide arrays to elucidate the alterations in gene expressions in these cells upon gestational nicotine exposure. Microarray analysis identified a set of 135 genes as significantly differentially expressed between DA and non-DA neurons. Some of the genes were found to be related to neurological disease pathways, such as Alzheimer’s disease, Parkinson’s disease and Huntington’s disease. Significantly up-/down-regulated genes found in DA neurons were mostly related to G-protein-coupled protein receptor signaling and developmental processes. These alterations in gene expressions may explain, partially at least, the possible pathological mechanisms for the diseases induced by maternal smoking.





Similar content being viewed by others
References
Alcaro A, Huber R, Panksepp J (2007) Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev 56:283–321. doi:10.1016/j.brainresrev.2007.07.014
Bardy AH, Seppala T, Lillsunde P, Kataja JM, Koskela P, Pikkarainen J, Hiilesmaa VK (1993) Objectively measured tobacco exposure during pregnancy—neonatal effects and relation to maternal smoking. Br J Obstet Gynaecol 100:721–726. doi:10.1111/j.1471-0528.1993.tb14262.x
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G (1989) The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic-activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning-tasks in rats. J Nutr 119:1880–1892
Brischoux F, Fellmann D, Risold PY (2001) Ontogenetic development of the diencephalic MCH neurons: a hypothalamic ‘MCH area’ hypothesis. Eur J Neurosci 13:1733–1744. doi:10.1046/j.0953-816x.2001.01552.x
Britt JP, McGehee DS (2008) Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens. J Neurosci 28:1672–1681. doi:10.1523/jneurosci.4275-07.2008
Brouillet E, Conde F, Beal MF, Hantraye P (1999) Replicating Huntington’s disease phenotype in experimental animals. Prog Neurobiol 59:427–468. doi:10.1016/s0301-0082(99)00005-2
Browne CJ, Sharma N, Waters KA, Machaalani R (2010) The effects of nicotine on the alpha-7 and beta-2 nicotinic acetylcholine receptor subunits in the developing piglet brainstem. Int J Dev Neurosci 28:1–7. doi:10.1016/j.ijdevneu.2009.10.005
Buchberger A, Howard MJ, Proctor M, Bycroft M (2001) The UBX domain: a widespread ubiquitin-like module. J Mol Biol 307:17–24. doi:10.1006/jmbi.2000.4462
Buka SL, Shenassa ED, Niaura R (2003) Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry 160:1978–1984. doi:10.1176/appi.ajp.160.11.1978
Cajigas IJ, Will T, Schuman EM (2010) Protein homeostasis and synaptic plasticity. EMBO J 29:2746–2752. doi:10.1038/emboj.2010.173
Carpenter AC, Saborido TP, Stanwood GD (2012) Development of hyperactivity and anxiety responses in dopamine transporter-deficient mice. Dev Neurosci 34:250–257. doi:10.1159/000336824
Chandrasekaran K, Hatanpaa K, Rapoport SI, Brady DR (1997) Decreased expression of nuclear and mitochondrial DNA-encoded genes of oxidative phosphorylation in association neocortex in Alzheimer disease. Brain Res Mol Brain Res 44:99–104
Chen H, Parker SL, Matta SG, Sharp BM (2005) Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. Eur J Neurosci 22:380–388. doi:10.1111/j.1460-9568.2005.04229.x
Christianson JC, Green WN (2004) Regulation of nicotinic receptor expression by the ubiquitin–proteasome system. EMBO J 23:4156–4165. doi:10.1038/sj.emboj.7600436
Cornelius MD, Day NL (2009) Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol 22:121–125. doi:10.1097/WCO.0b013e328326f6dc
DiAntonio A, Hicke L (2004) Ubiquitin-dependent regulation of the synapse. Annu Rev Neurosci 27:223–246. doi:10.1146/annurev.neuro.27.070203.144317
Ding S, Wei W, Zhou F-M (2011) Molecular and functional differences in voltage-activated sodium currents between GABA projection neurons and dopamine neurons in the substantia nigra. J Neurophysiol 106:3019–3034. doi:10.1152/jn.00305.2011
Ekblad M, Korkeila J, Parkkola R, Lapinleimu H, Haataja L, Lehtonen L, Grp PS (2010) Maternal smoking during pregnancy and regional brain volumes in preterm infants. J Pediatr 156:185-U140. doi:10.1016/j.jpeds.2009.07.061
Enslen M, Milon H, Malnoe A (1991) Effect of low intake of n-3 fatty-acids during development on brain phospholipid fatty-acid composition and exploratory-behavior in rats. Lipids 26:203–208. doi:10.1007/bf02543972
Frances H, Monier C, Clement M, Lecorsier A, Debray M, Bourre JM (1996) Effect of dietary alpha-linolenic acid deficiency on habituation. Life Sci 58:1805–1816. doi:10.1016/0024-3205(96)00164-6
Fried PA, Watkinson B, Gray R (1992) A follow-up-study of attentional behavior in 6-year-old children exposed prenatally to marijuana, cigarettes, and alcohol. Neurotoxicol Teratol 14:299–311. doi:10.1016/0892-0362(92)90036-a
Fukuyama R, Hatanpaa K, Rapoport SI, Chandrasekaran K (1996) Gene expression of ND4, a subunit of complex I of oxidative phosphorylation in mitochondria, is decreased in temporal cortex of brains of Alzheimer’s disease patients. Brain Res 713:290–293. doi:10.1016/0006-8993(95)01517-5
Gaimarri A, Moretti M, Riganti L, Zanardi A, Clementi F, Gotti C (2007) Regulation of neuronal nicotinic receptor traffic and expression. Brain Res Rev 55:134–143. doi:10.1016/j.brainresrev.2007.02.005
Gainetdinov RR, Caron MG (2000) An animal model of attention deficit hyperactivity disorder. Mol Med Today 6:43–44. doi:10.1016/s1357-4310(99)01616-0
Garrido R, Mattson MP, Hennig B, Toborek M (2001) Nicotine protects against arachidonic-acid-induced caspase activation, cytochrome c release and apoptosis of cultured spinal cord neurons. J Neurochem 76:1395–1403. doi:10.1046/j.1471-4159.2001.00135.x
Giros B, Caron MG (1993) Molecular characterization of the dopamine transporter. Trends Pharmacol Sci 14:43–49. doi:10.1016/0165-6147(93)90029-j
Gizer IR, Ficks C, Waldman ID (2009) Candidate gene studies of ADHD: a meta-analytic review. Hum Genet 126:51–90. doi:10.1007/s00439-009-0694-x
Haglund K, Dikic I (2005) Ubiquitylation and cell signaling. EMBO J 24:3353–3359. doi:10.1038/sj.emboj.7600808
Harrod SB, Lacy RT, Zhu J, Hughes BA, Perna MK, Brown RW (2011) Gestational IV nicotine produces elevated brain-derived neurotrophic factor in the mesocorticolimbic dopamine system of adolescent rat offspring. Synapse 65:1382–1392. doi:10.1002/syn.20975
Herrmann M, King K, Weitzman M (2008) Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr Opin Pediatr 20:184–190. doi:10.1097/MOP.0b013e3282f56165
Hill SY, Lowers L, Locke-Wellman J, Shen SA (2000) Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. J Stud Alcohol 61:661–668
Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH (2010) Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron 65:643–656. doi:10.1016/j.neuron.2010.02.012
Huang DW, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. doi:10.1093/nar/gkn923
Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. doi:10.1038/nprot.2008.211
Hubert GW, Jones DC, Moffett MC, Rogge G, Kuhar MJ (2008) CART peptides as modulators of dopamine and psychostimulants and interactions with the mesolimbic dopaminergic system. Biochem Pharmacol 75:57–62. doi:10.1016/j.bcp.2007.07.028
Jaworski JN, Jones DC (2006) The role of CART in the reward/reinforcing properties of psychostimulants. Peptides 27:1993–2004. doi:10.1016/j.peptides.2006.03.034
Jentsch S, Rumpf S (2007) Cdc48 (p97): a ‘molecular gearbox’ in the ubiquitin pathway? Trends Biochem Sci 32:6–11. doi:10.1016/j.tibs.2006.11.005
Johansen EB, Killeen PR, Russell VA, Tripp G, Wickens JR, Tannock R, Williams J, Sagvolden T (2009) Origins of altered reinforcement effects in ADHD. Behav Brain Funct. doi:10.1186/1744-9081-5-7
Jones S, Kornblum JL, Kauer JA (2000) Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J Neurosci 20:5575–5580
Kahn RS, Khoury J, Nichols WC, Lanphear BP (2003) Role of dopamine transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. J Pediatr 143:104–110. doi:10.1016/s0022-3476(03)00208-7
Kane VB, Fu YT, Matta SG, Sharp BM (2004) Gestational nicotine exposure attenuates nicotine-stimulated dopamine release in the nucleus accumbens shell of adolescent Lewis rats. J Pharmacol Exp Ther 308:521–528. doi:10.1124/jpet.103.059899
Kauer JA, Malenka RC (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8:844–858. doi:10.1038/nrn2234
Keller JN, Hanni KB, Markesbery WR (2000) Impaired proteasome function in Alzheimer’s disease. J Neurochem 75:436–439. doi:10.1046/j.1471-4159.2000.0750436.x
Kheradpezhouh M, Shavali S, Ebadi M (2003) Salsolinol causing parkinsonism activates endoplasmic reticulum-stress signaling pathways in human dopaminergic SK-N-SH cells. Neurosignals 12:315–324. doi:10.1159/000075314
Kim J, Moody JP, Edgerly CK, Bordiuk OL, Cormier K, Smith K, Beal MF, Ferrante RJ (2010) Mitochondrial loss, dysfunction and altered dynamics in Huntington’s disease. Hum Mol Genet 19:3919–3935. doi:10.1093/hmg/ddq306
Kimmel HL, Gong WH, Dall Vechia S, Hunter RG, Kuhar MJ (2000) Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55-102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther 294:784–792
Klann E, Antion MD, Banko JL, Hou LF (2004) Synaptic plasticity and translation initiation. Learn Mem 11:365–372. doi:10.1101/lm.79004
Klink R, d’Exaerde AD, Zoli M, Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21:1452–1463
Kopito RR, Ron D (2000) Conformational disease. Nat Cell Biol 2:E207–E209. doi:10.1038/35041139
Korotkova TM, Ponomarenko AA, Brown RE, Haas HL (2004) Functional diversity of ventral midbrain dopamine and GABAergic neurons. Mol Neurobiol 29:243–259. doi:10.1385/mn:29:3:243
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645. doi:10.1101/gr.092759.109
Kurtoglu S, Gunes T, Koklu E, Bastug O, Canoz O, Kula M, Bastug F, Gunes I (2007) Influence of maternal nicotine exposure on neonatal rat bone: protective effect of pentoxifylline. Exp Biol Med 232:398–405
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980. doi:10.1126/science.6823561
Lauro C, Di Angelantonio S, Cipriani R, Sobrero F, Antonilli L, Brusadin V, Ragozzino D, Limatola C (2008) Activity of adenosine receptors type 1 is required for CX(3)CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol 180:7590–7596
le Blanc LMP, van Lieshout AWT, Adema GJ, van Riel P, Verbeek MM, Radstake T (2006) CXCL16 is elevated in the cerebrospinal fluid versus serum and in inflammatory conditions with suspected and proved central nervous system involvement. Neurosci Lett 397:145–148. doi:10.1016/j.neulet.2005.12.029
Li LB, Chen NH, Ramamoorthy S, Chi LM, Cui XN, Wang LJC, Reith MEA (2004) The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem 279:21012–21020. doi:10.1074/jbc.M311972200
Li Q, Lu G, Antonio GE, Mak YT, Rudd JA, Fan M, Yew DT (2007) The usefulness of spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem Int 50:848–857. doi:10.1016/j.neuint.2007.02.005
Lim DK, Park SH, Choi WJ (2000) Subacute nicotine exposure in cultured cerebellar cells increased the release and uptake of glutamate. Arch Pharmacal Res 23:488–494. doi:10.1007/bf02976578
Lim KL, Tan JMM (2007) Role of the ubiquitin proteasome system in Parkinson’s disease. BMC Biochem. doi:10.1186/1471-2091-8-S1-S13
Lopez-Hidalgo M, Salgado-Puga K, Alvarado-Martinez R, Cristina Medina A, Prado-Alcala RA, Garcia-Colunga J (2012) Nicotine uses neuron-glia communication to enhance hippocampal synaptic transmission and long-term memory. Plos One. doi:10.1371/journal.pone.0049998
Louis JCM (1996) Methods of preventing neuron degeneration and promoting neuron regeneration. Amgen
Luck W, Nau H (1984) Nicotine and cotinine concentrations in serum and milk of nursing smokers. Br J Clin Pharmacol 18:9–15
Margolis EB, Lock H, Hjelmstad GO, Fields HL (2006) The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol Lond 577:907–924. doi:10.1113/jphysiol.2006.117069
Meyer H (2012) p97 complexes as signal integration hubs. BMC Biol. doi:10.1186/1741-7007-10-48
Navarro HA, Seidler FJ, Whitmore WL, Slotkin TA (1988) Prenatal exposure to nicotine via maternal infusions-effects on development of catecholamine systems. J Pharmacol Exp Ther 244:940–944
Neuman RJ, Lobos E, Reich W, Henderson CA, Sun L-W, Todd RD (2007) Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biol Psychiatry 61:1320–1328. doi:10.1016/j.biopsych.2006.08.049
Nisell M, Marcus M, Nomikos GG, Svensson TH (1997) Differential effects of acute and chronic nicotine on dopamine output in the core and shell of the rat nucleus accumbens. J Neural Transm 104:1–10. doi:10.1007/bf01271290
Nishizaki T, Nomura T, Matsuoka T, Enikolopov G, Sumikawa K (1999) Arachidonic acid induces a long-lasting facilitation of hippocampal synaptic transmission by modulating PKC activity and nicotinic ACh receptors. Mol Brain Res 69:263–272. doi:10.1016/s0169-328x(99)00117-5
Owens SD, Innis SM (1999) Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alpha-linolenic acid deficient diet in formula-fed piglets. J Nutr 129:2088–2093
Parker WD, Parks J, Filley CM, Kleinschmidtdemasters BK (1994) Electron-transport chain defects in Alzheimers-disease brain. Neurology 44:1090–1096
Parker WD, Swerdlow RH (1998) Mitochondrial dysfunction in idiopathic Parkinson disease. Am J Hum Genet 62:758–762. doi:10.1086/301812
Pauly JR, Slotkin TA (2008) Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr 97:1331–1337. doi:10.1111/j.1651-2227.2008.00852.x
Pierce RC, Kumaresan V (2006) The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30:215–238. doi:10.1016/j.neubiorev.2005.04.016
Rezvani K, Teng Y, Shim D, De Biasi M (2007) Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J Neurosci 27:10508–10519. doi:10.1523/jneurosci.3353-07.2007
Ribary U, Lichtensteiger W (1989) Effects of acute and chronic prenatal nicotine treatment on central catecholamine systems of male and female rat fetuses and offspring. J Pharmacol Exp Ther 248:786–792
Richardson SA, Tizabi Y (1994) Hyperactivity in the offspring of nicotine-treated rats—role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol Biochem Behav 47:331–337. doi:10.1016/0091-3057(94)90018-3
Rosito M, Deflorio C, Limatola C, Trettel F (2012) CXCL16 orchestrates adenosine A(3) receptor and MCP-1/CCL2 activity to protect neurons from excitotoxic cell death in the CNS. J Neurosci 32:3154–3163. doi:10.1523/jneurosci.4046-11.2012
Roy TS, Sabherwal U (1998) Effects of gestational nicotine exposure on hippocampal morphology. Neurotoxicol Teratol 20:465–473. doi:10.1016/s0892-0362(97)00137-2
Roy TS, Seidler FJ, Slotkin TA (2002) Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. J Pharmacol Exp Ther 300:124–133. doi:10.1124/jpet.300.1.124
Roza SJ, Verburg BO, Jaddoe VWV, Hofman A, Mackenbach JP, Steegers EAP, Witteman JCM, Verhulst FC, Tiemeier H (2007) Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. Eur J Neurosci 25:611–617. doi:10.1111/j.1460-9568.2007.05393.x
Salihu HM, Wilson RE, Alio AP, Kirby RS (2008) Advanced maternal age and risk of antepartum and intrapartum stillbirth. J Obstet Gynaecol Res 34:843–850. doi:10.1111/j.1447-0756.2008.00855.x
Sastry PS (1985) Lipids of nervous-tissue-composition and metabolism. Prog Lipid Res 24:69–176. doi:10.1016/0163-7827(85)90011-6
Schneider T, Ilott N, Brolese G, Bizarro L, Asherson PJE, Stolerman IP (2011) Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology 36:1114–1125. doi:10.1038/npp.2010.249
Schuberth C, Buchberger A (2008) UBX domain proteins: major regulators of the AAA ATPase Cdc48/p97. Cell Mol Life Sci 65:2360–2371. doi:10.1007/s00018-008-8072-8
Selkoe DJ (2003) Folding proteins in fatal ways. Nature 426:900–904. doi:10.1038/nature02264
Sershen H, Mason MF, Reith MEA, Hashim A, Lajtha A (1986) Effect of amphetamine on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity in mice. Neuropharmacology 25:927–930. doi:10.1016/0028-3908(86)90022-5
Slotkin TA, Cho H, Whitmore WL (1987) Effects of prenatal nicotine exposure on neuronal development—selective actions on central and peripheral catecholaminergic pathways. Brain Res Bull 18:601–611. doi:10.1016/0361-9230(87)90130-4
Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Lopez-Real AM, Labandeira-Garcia JL (2002) Effects of (−)-nicotine and (−)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson’s disease. Biochem Pharmacol 64:125–135. doi:10.1016/s0006-2952(02)01070-5
Sprecher H, Luthria DL, Mohammed BS, Baykousheva SP (1995) Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res 36:2471–2477
Sutton MA, Schuman EM (2006) Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127:49–58. doi:10.1016/j.cell.2006.09.014
Swan GE, Lessov-Schlaggar CN (2007) The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev 17:259–273. doi:10.1007/s11065-007-9035-9
Thapar A, van den Bree M, Fowler T, Langley K, Whittinger N (2006) Predictors of antisocial behaviour in children with attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry 15:118–125. doi:10.1007/s00787-006-0511-1
Ungless MA, Whistler JL, Malenka RC, Bonci A (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411:583–587. doi:10.1038/35079077
Vanbockstaele EJ, Pickel VM (1995) GABA-containing neurons in the ventral tegmental area project to the nucleus-accumbens in rat-brain. Brain Res 682:215–221. doi:10.1016/0006-8993(95)00334-m
Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, Paya CV (2002) G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol 169:5546–5554
Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL (1997) Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry 54:670–676
Wang J, Duncan D, Shi Z, Zhang B (2013) WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res 41:W77–W83. doi:10.1093/nar/gkt439
Ward SG, Westwick J (1998) Chemokines: understanding their role in T-lymphocyte biology. Biochem J 333:457–470
Weissman MM, Warner V, Wickramaratne PJ, Kandel DB (1999) Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry 38:892–899. doi:10.1097/00004583-199907000-00020
White SR, Lauring B (2007) AAA+ ATPases: achieving diversity of function with conserved machinery. Traffic 8:1657–1667. doi:10.1111/j.1600-0854.2007.00642.x
Wise RA (1985) The anhedonia hypothesis—mark-III. Behav Brain Sci 8:178–184
Yamamoto BK, Novotney S (1998) Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem 71:274–280
Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP (2002) Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. doi:10.1093/nar/30.4.e15
Yi JJ, Ehlers MD (2007) Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacol Rev 59:14–39. doi:10.1124/pr.59.1.4
Zhang B, Kirov S, Snoddy J (2005) WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33:W741–W748. doi:10.1093/nar/gki475
Zhang H, Li S, Wang M, Vukusic B, Pristupa ZB, Liu F (2009) Regulation of dopamine transporter activity by carboxypeptidase E. Molecular Brain. doi:10.1186/1756-6606-2-10
Zhang TA, Placzek AN, Dani JA (2010) In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology 59:431–436. doi:10.1016/j.neuropharm.2010.06.004
Zhu J, Apparsundaram S, Dwoskin LP (2009) Nicotinic receptor activation increases H-3 dopamine uptake and cell surface expression of dopamine transporters in rat prefrontal cortex. J Pharmacol Exp Ther 328:931–939. doi:10.1124/jpet.108.147025
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Timeline for the sample collections and presentation of samples used in this study. (PDF 98 kb)
Supplementary Fig. 2
Heatmap displaying the expression fold changes of the 135 differentially expressed genes in DA vs non-DA neurons. (PDF 93 kb)
Supplementary Fig. 3
Compact diagram of working flow of WebGestalt program. (PDF 76 kb)
Rights and permissions
About this article
Cite this article
Kanlikilicer, P., Zhang, D., Dragomir, A. et al. Gene expression profiling of midbrain dopamine neurons upon gestational nicotine exposure. Med Biol Eng Comput 55, 467–482 (2017). https://doi.org/10.1007/s11517-016-1531-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-016-1531-8