Abstract
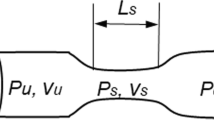
Aging and some pathologies such as arterial hypertension, diabetes, hyperglycemia, and hyperinsulinemia cause some geometrical and mechanical changes in the aortic valve microstructure which contribute to the development of aortic stenosis (AS). Because of the high rate of mortality and morbidity, assessing the impact and progression of this disease is essential. Systolic transvalvular pressure gradient (TPG) and the effective orifice area are commonly used to grade the severity of valvular dysfunction. In this study, a theoretical model of the transient viscous blood flow across the AS is derived by taking into account the aorta compliance. The derived relation of the new TPG is expressed in terms of clinically available surrogate variables (anatomical and hemodynamic data). The proposed relation includes empirical constants which need to be empirically determined. We used a numerical model including an anatomically 3D geometrical model of the aortic root including the sinuses of Valsalva for their identification. The relation was evaluated using clinical values of pressure drops for cases for which the modified Gorlin equation is problematic (low flow, low gradient AS).






Similar content being viewed by others
Abbreviations
- A d :
-
Flow cross-sectional area downstream of the stenosis, cm2
- A u :
-
Flow cross-sectional area upstream of the stenosis, cm2
- a :
-
Vessel radius, cm
- C :
-
Vessel compliance, cm/Barye
- E :
-
Young’s modulus of the vessel, Barye
- EOA:
-
Flow effective orifice area, cm2
- \(\vec{F}\) :
-
Body and surface force vector, g cm/s2
- \(g(\frac{{A_{\text{d}} }}{\text{EOA}})\) :
-
Function of the areas ratio, dimensionless
- h :
-
Vessel thickness, cm
- k c :
-
Empirical constant in the convective pressure loss term, dimensionless
- k p :
-
Empirical constant in the pressure loss term due to the vessel compliance, Barye
- k v :
-
Empirical constant in the viscous pressure loss, dimensionless
- L 23 :
-
Distance between two referenced positions downstream and upstream of the stenosis, cm
- \(\vec{n}\) :
-
Outward pointing normal unit vector to the body surface, dimensionless
- p :
-
Blood flow pressure, dyn/cm2
- Q :
-
Volume flow rate, cm3/s
- u :
-
Blood flow velocity, cm/s
- \(\vec{V}\) :
-
Fluid velocity vector, cm/s
- λ :
-
Parameter defined as \(\frac{{L_{23} }}{{A_{\text{d}} }} + \int\limits_{{x_{1} }}^{{x_{2} }} {\frac{{{\text{d}}x}}{A}}\), cm−1
- ζ :
-
Empirical constant, dimensionless
- ρ :
-
Blood density, g/cm3
- μ :
-
Dynamic viscosity of blood, g/cm s
- v :
-
Kinematic viscosity of blood, cm2/s
- ω :
-
Heart frequency, Hz
- τ :
-
Vessel wall shear stress, dyn/cm2
- ∆p :
-
Pressure gradient across stenosis, dyn/cm2
- ∆p C :
-
Convective component of the pressure gradient across stenosis, dyn/cm2
- ∆p Co :
-
Pressure gradient component due to the vessel distensibility, dyn/cm2
- ∆p L :
-
Pressure gradient component due to the local inertia, dyn/cm2
- ∆p V :
-
Viscous component of the pressure gradient across stenosis, dyn/cm2
- σ ij :
-
Cauchy stress tensor, dyn/cm2
- V i :
-
Material velocity vector, cm/s
- V MJ :
-
Framework velocity, cm/s
- C :
-
Stiffness tensor of the vessel wall material, dyn/cm2
- ε :
-
Strain tensor of the vessel wall material, dimensionless
- V f :
-
Vector containing the fluid unknowns
- V s :
-
Vector containing the solid unknowns
- Γfs :
-
Common solid–fluid interface
References
Ask P, Loyd D, Wranne B (1986) Regurgitant flow through heart valves: a hydraulic model applicable to ultrasound Doppler measurements. Med Biol Eng Comput 24:643–646
Baumgartner H (2012) Low-flow, low-gradient aortic stenosis with preserved ejection fraction still a challenging condition. J Am Coll Cardiol 60:1268–1270
Baumgartner H, Stefenelli T, Niederberger J, Schima H, Maurer G (1999) “Overestimation” of catheter gradients by Doppler ultrasound in patients with aortic stenosis: a predictable manifestation of pressure recovery. J Am Coll Cardiol 33:1655–1661
Ben-Israel A, Greville TNE (2003) Generalized inverses: theory and applications, 2nd edn. Springer, New York
Bermejo J, Antoranz JC, Burwash IG, Alvarez JL, Moreno M, Garcia-Fernandez MA, Otto CM (2002) In-vivo analysis of the instantaneous transvalvular pressure difference-flow relationship in aortic valve stenosis: implications of unsteady fluid-dynamics for the clinical assessment of disease severity. J Heart Valve Dis 11:557–566
Bonow RO, Carabello B, De Leon AC Jr, Edmunds LH Jr, Fedderly BJ, Freed MD, Gaasch WH, McKay CR, Nishimura RA, O’Gara PT, O’Rourke RA, Rahimtoola SH, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A Jr, Gibbons RJ, Russell RO, Ryan TJ, Smith SC Jr (1998) Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation 98:1949–1984
Cannon SR, Richards KL, Crawford M (1985) Hydraulic estimation of stenotic orifice area: a correction of the Gorlin formula. Circulation 71:1170–1178
Capelli C, Bosi GM, Cerri E, Nordmeyer J, Odenwald T, Bonhoeffer P, Migliavacca F, Taylor AM, Schievano S (2012) Patient-specific simulations of transcatheter aortic valve stent implantation. Med Biol Eng Comput 50:183–192
Carmody CJ, Burriesci G, Howard IC, Patterson EA (2006) An approach to the simulation of fluid–structure interaction in the aortic valve. J Biomech 39:158–169
Choudhury N, Bouchot O, Rouleau L, Tremblay D, Cartier R, Butany J, Mongrain R, Leask RL (2009) Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Am J Cardiovasc Pathol 18:83–91
Clark C (1978) Relation between pressure difference across the aortic valve and left ventricular outflow. Cardiovasc Res 12:276–287
de Tullio MD, Afferrante L, Demelio G, Pascazio G, Verzicco R (2011) Fluid–structure interaction of deformable aortic prostheses with a bileaflet mechanical valve. J Biomech 44:1684–1690
Dettmer W, Perić D (2006) A computational framework for fluid–structure interaction: finite element formulation and applications. Comput Methods Appl Mech Eng 195(41):5754–5779
Dobrin PB (1991) Poststenotic dilatation. Surg Gynecol Obstet 172:503–508
Farhat C, Lesoinne M, Le Tallec P (1998) Load and motion transfer algorithms for fluid/structure interaction problems with non-matching discrete interfaces: momentum and energy conservation, optimal discretization and application to aeroelasticity. Comput Methods Appl Mech Eng 157(1):95–114
Fernández MA, Gerbeau JF, Grandmont C (2007) A projection semi-implicit scheme for the coupling of an elastic structure with an incompressible fluid. Int J Numer Meth Eng 69:794–821
Fung Y (1998) Biomechanics: circulation, 2nd edn. Springer, New York
Garcia D, Pibarot P, Durand LG (2005) Analytical modeling of the instantaneous pressure gradient across the aortic valve. J Biomech 38:1303–1311
Gerhart PM (1992) Fundamentals of fluid mechanics, 2nd edn. Addison-Wesley Pub. Co., Reading
Gorlin R, Gorlin SG (1951) Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts I. Am Heart J 41:1–29
Hallquist JO (2006) LS-DYNA theory manual. Livermore Software Technology Corporation, CA, USA, ISBN 0-9778540-0-0
Heinrich R, Fontaine A, Grimes R, Sidhaye A, Yang S, Moore K, Levine R, Yoganathan A (1996) Experimental analysis of fluid mechanical energy losses in aortic valve stenosis: importance of pressure recovery. Ann Biomed Eng 24:685–694
Idelsohn SR, Del Pin F, Rossi R, Oñate E (2009) Fluid–structure interaction problems with strong added-mass effect. Int J Numer Methods Eng 80:1261–1294
Krishnamurthy G, Itoh A, Bothe W, Swanson JC, Kuhl E, Karlsson M, Craig Miller D, Ingels NB Jr (2009) Stress-strain behavior of mitral valve leaflets in the beating ovine heart. J Biomech 42:1909–1916
Kunzelman KS, Einstein DR, Cochran RP (2007) Fluid–structure interaction models of the mitral valve: function in normal and pathological states. Philos Trans R Soc Lond B Biol Sci 362:1393–1406
Livermore Software Technology Corporation (LSTC) (2013) Theory manual incompressible fluid solver in LS-DYNA, Livermore, CA
Miller DS (1990) Internal flow systems. BHRA (Information Services), Cranfield
Nobari S, Mongrain R, Leask R, Cartier R (2013) The effect of aortic wall and aortic leaflet stiffening on coronary hemodynamic: a fluid–structure interaction study. Med Biol Eng Comput 51(8):923–936
Olsen MH, Wachtell K, Bella JN, Liu JE, Boman K, Gerdts E, Papademetriou V, Nieminen MS, Rokkedal J, Dahlof B, Devereux RB (2004) Effect of losartan versus atenolol on aortic valve sclerosis (a LIFE substudy). Am J Cardiol 94:1076–1080
Olsen MH, Wachtell K, Bella JN, Gerdts E, Palmieri V, Nieminen MS, Smith G, Ibsen H, Devereux RB, LIFE substudy (2005) Aortic valve sclerosis relates to cardiovascular events in patients with hypertension (a LIFE substudy). Am J Cardiol 95:132–136
Otto CM (2004) Why is aortic sclerosis associated with adverse clinical outcomes? J Am Coll Cardiol 43:176–178
Pappano AJ, Wier WG (2012) Cardiovascular physiology: mosby physiology monograph series, 10th edn. Elsevier Mosby, Philadelphia
Ranga A, Bouchot O, Mongrain R, Ugolini P, Cartier R (2006) Computational simulations of the aortic valve validated by imaging data: evaluation of valve-sparing techniques. Interact CardioVasc Thorac Surg 5:373–378
Ross J Jr (1985) Afterload mismatch in aortic and mitral valve disease: implications for surgical therapy. J Am Coll Cardiol 5:811–826
Schlichting H (1960) Boundary layer theory, 4th edn. McGraw Hill, New York
Shavelle D, Otto C (2000) Aortic stenosis. In: Crawford MH, Dimarco JP (eds) Cardiology. Mosby, London, pp 9.1–9.10
Slørdahl SA, Solbakken JE, Piene H, Angelsen BAJ, Rossvoll O, Samstad SO (1990) Quantification of aortic regurgitation by Doppler echocardiography: a new method evaluated in pigs. Med Biol Eng Comput 28:300–305
Thubrikar M, Piepgrass WC, Bosher LP, Nolan SP (1980) The elastic modulus of canine aortic valve leaflets in vivo and in vitro. Circ Res 47:792–800
Young DF, Tsai FY (1973) Flow characteristics in models of arterial stenoses I. Steady flow. J Biomech 6:395–410
Young DF, Tsai FY (1973) Flow characteristics in models of arterial stenoses II. Unsteady flow. J Biomech 6:547–559
Acknowledgments
We are thankful to the support of McGill Engineering Doctoral Award (MEDA), Natural Sciences and Engineering Research Council of Canada (NSERC) and Montreal Heart Institute (MHI). We would also like to thank Mr. Facundo Del Pin, a scientist at Livermore Software Technology Corporation for developing the ICFD solver. This work was made possible by the facilities of the Shared Hierarchical Academic Research Computing Network (SHARCNET: www.sharcnet.ca) and Compute/Calcul Canada.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
For a distensible wall with fixed EOA, the flow disturbance downstream of the stenosis causes a change in the cross-sectional area by an amount dA.Then, for the compliant vessel, the convective pressure loss becomes:
If the vessel is modeled with a thin-walled cylinder obeying Hooke’s law (assuming physiological deformation), since the longitudinal stress is much smaller that the circumferential one, then
where e θθ is the circumferential strain, a the radius of the vessel, a 0 the initial radius, E the Young’s modulus of the wall material, and h the wall thickness. Then, the cross-sectional variation in Eq. (32) can be expressed as:
Hence, by expanding Eq. (32), neglecting the small higher-order terms and using Eqs. (33) and (34), the effect of the wall compliance in pressure loss can be expressed as:
The first term in the right-hand side (∆p Rigid) is pressure loss caused by convective inertia in the rigid vessel, while the second term (∆p Co) corresponds to the contribution of the vessel compliance to convective pressure loss as the following:
where dp is the pressure variation in the aorta. This term can be scaled as a fraction of the pulse pressure by introducing an empirical constant k p which has the dimension of the pressure. Hence, by including the effect of all constant coefficients of Eq. (36) in k p, it can be simplified as:
Rights and permissions
About this article
Cite this article
Mohammadi, H., Cartier, R. & Mongrain, R. Derivation of a simplified relation for assessing aortic root pressure drop incorporating wall compliance. Med Biol Eng Comput 53, 241–251 (2015). https://doi.org/10.1007/s11517-014-1228-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-014-1228-9