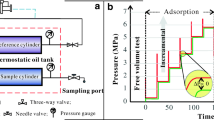
Abstract
CO2 sorption and diffusion in coal are closely related to the occurrence of coal and gas outburst, geological sequestration of CO2 in coalbeds, and enhancing coalbed methane recovery by injecting CO2. Hence, it is significant to investigate the sorption properties and diffusion models of CO2 in coal. Here we used a newly designed experimental apparatus at Peking University to investigate the sorption and diffusion properties of CO2 in natural coal samples from Dashucun Mine and Wutongzhuang Mine in Handan city, Hebei province, and Jinhuagong Mine in Datong city, Shanxi province, and obtained CO2 sorption isotherms and diffusivity models. The results indicate that, in a certain pressure range, CO2 sorption isotherms for the coal samples are consistent with the Langmuir model, which assumes that monolayer sorption occurs at the interface between coal matrix and CO2 molecules, and the sorption isotherms feature nonstandard hyperbolas in mathematics. At the same pressure and temperature, as the vitrinite content increases, coal adsorbs more CO2 molecules. The relation between the sorption capacity and the coal rank may be described as a “U-type” trend, and medium rank coal has the least sorption capacity. The bulk diffusivity of CO2 in coal is not constant; in the range of CO2 mass fraction greater than 1%, it increases roughly linearly with increasing mass fraction of CO2 adsorbed (or CO2 partial pressure) in coal. CO2 diffusivity in coal is approximately 10−4 to 10−2 mm2/s in magnitudes, and the diffusivity ranges in coal samples are 3×10−4 to 8×10−3 mm2/s from Dashucun Mine, 2×10−4 to 4×10−3 mm2/s from Wutongzhuang Mine, and 2×10−4 to 4×10−3 mm2/s from Jinhuagong Mine. The results of the CO2 sorption and diffusion study can be applied to help predict and prevent coal and gas outburst as well as to evaluate the feasibility in geological sequestration of CO2 and to enhance coalbed methane recovery.
Similar content being viewed by others
References
Guan P, Wang H Y, Zhang Y X. Mechanism of instantaneous coal outbursts. Geology, 2009, 37: 915–918
Bodziony J, Lama R D. Sudden outbursts of gas and coal in underground coal mines. Australian Coal Association Research Program Report C4034, 1996. 677
Zhou S N, Lin B Q. Coal Seams Gas Sorption and Flow Theory (in Chinese). Beijing: Coal Industry Press, 1997
Gunter W D, Wong S, Cheel D B, et al. Large CO2 sinks: Their role in the mitigation of greenhouse gases from an international, national (Canadian) and provincial (Alberta) perspective. Appl Energ, 1998, 61: 209–227
Gentzis T. Subsurface sequestration of carbon dioxide—An overview from an Alberta (Canada) perspective. Int J Coal Geol, 2000, 43: 287–305
Van Bergen F, Pagnier H, Krzystolik P. Field experiments of enhanced coalbed methane-CO2 in the upper Silesian basin of Poland. Environ Geosci, 2006, 13: 201–224
Kronimus A, Busch A, Alles S, et al. A preliminary evaluation of the CO2 storage potential in unminable coal seams of the Munster Cretaceous Basin, Germany. Int J Greenh Gas Con, 2008, 2: 329–341
White C M, Smith D H, Jones K L, et al. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery—A review. Energ Fuel, 2005, 19: 659–724
Wang E Y, He X Q, Lin H Y. adsorption statement of gas in coal (in Chinese). Coal Eng, 1996, 5: 12–16
Zhang L, He X Q, Wang E Y, et al. Study of absorptive characteristics of coal (in Chinese). J Taiyuan Univ Tech, 2001, 32: 449–451
Xie J L, Guo Y Y, Wu S Y. Study of adsorption of methane on coal at normal temperature (in Chinese). J Taiyuan Univ Tech, 2004, 35: 562–564
Sang S X, Zhu Y M, Zhang J, et al. The mechanism of gas adsorption in coal (II)—the physical process and theory model (in Chinese). Nat Gas Ind, 2005, 25: 16–18
Jiang W P, Cui Y J, Zhong L W, et al. Quantum chemical study on coal surface interacting with CH4 and H2O (in Chinese). Nat Gas Geosci, 2007, 18: 576–579
Zhang S Y, Sang S X, Yang Z G. Mechanism analysis on the effect of liquid water on coal adsorbing methane (in Chinese). J Chin Univ Min Tech, 2009, 38: 707–712
Jiang W P. Microscopic mechanism study on the influence of coal rank on adsorption capacity (in Chinese). Chin Coalbed Methane, 2009, 6: 19–22
Sang S X, Zhu Y M, Zhang J, et al. Influence of liquid water on coalbed methane adsorption: An experimental research on coal reservoirs in the south of Qingshui Basin. Chin Sci Bull, 2005, 50(Suppl.1): 79–85
Zhang L P, Su X B, Zeng R S. Discussion on the controlling effects of coal properties on coal adsorption capacity (in Chinese). Acta Geol Sin, 2006, 80: 910–915
Zhang S Y, Sang S X. Mechanism of liquid water influencing on methane adsorption of coals with different ranks (in Chinese). Acta Geol Sin, 2008, 82: 1350–1354
Tang S H, Tang D Z, Yang Q. Binary-component gas adsorption isotherm experiments and their significance to exploitation of coalbed methane (in Chinese). J Chin Univ Geosci, 2004, 29: 219–223
Tang S H, Han D X. Exploitation potential evaluation to coalbed methane based on multi-component gas adsorption isotherms (in Chinese). J Chin Univ Min Tech, 2002, 31: 630–633
Tang S H, Yang Q, Tang D Z. Comparison between the experiment data of binary-component gas adsorption isotherms and the calculating results with extended-Langmuir equation (in Chinese). Geol Sci Technol Inf, 2003, 22: 68–70
Zhou S N. Mechanism of gas flow in coal seams (in Chinese). J Chin Coal Soc, 1990, 15: 15–24
Wu S Y. Preliminary approach on the low of gas diffusion and permeation on coal seams (in Chinese). J Taiyuan Univ Tech, 1994, 12: 259–263
Duan S M, Nie B S. The preliminary study on diffusion and permeation regularity of coal gas (in Chinese). J Taiyuan Univ Tech, 1998, 29: 413–416
Nie B S, He X Q, Wang E Y. Mechanism and modes of gas diffusion in coal seams (in Chinese). Chin Safety Sci J, 2000, 10: 24–28
Nie B S, Guo Y Y, Wu S Y, et al. Theoretical model of gas diffusion through coal particles and its analytical solution (in Chinese). J Chin Univ Min Tech, 2001, 30: 19–22
Nandi S P, Walker P L. Activated diffusion of methane in coal. Fuel, 1970, 49: 309–323
Harpalani S, Ouyang S. A new laboratory technique to estimate gas diffusion characteristics of coal. Int Coalbed Methane Symposium, May 3–7, 1999, Tuscaloosa, Alabama, 141–152
Saghafi A, Faiz M, Roberts D. CO2 storage and gas diffusivity properties of coals from sydney basin, australia. Int J Coal Geol, 2007, 70: 240–254
Saghafi A, Pinetown K L, Grobler P G, et al. CO2 storage potential of south african coals and gas entrapment enhancement due to igneous intrusions. Int J Coal Geol, 2008, 73: 74–87
Busch A, Gensterblum Y, Krooss B M. Methane and CO2 sorption and desorption measurements on dry argonne premium coals: Pure components and mixtures. Int J Coal Geol, 2003, 55: 205–224
Busch A, Gensterblum Y, Krooss B M, et al. Methane and carbon dioxide adsorption-diffusion experiments on coal: Upscaling and modeling. Int J Coal Geol, 2004, 60: 151–168
Mazumder S, Van Hemert P, Busch A, et al. Flue gas and pure CO2 sorption properties of coal: A comparative study. Int J Coal Geol, 2006, 67: 267–279
Siemons N, Busch A. Measurement and interpretation of supercritical CO2 sorption on various coals. Int J Coal Geol, 2007, 69: 229–242
Zhang Y X. Geochemical Kinetics. Boston: Princeton University Press, 2008. 224–227, 284–298, 418–434
Harpalani S, Chen G L. Estimation of changes in fracture porosity of coal with gas emission. Fuel, 1995, 74: 1491–1498
Harpalani S, Chen G. Influence of gas production induced volumetric strain on permeability of coal. Geotechnol Geol Eng, 1997, 15: 303–325
Van Bergen F, Spiers C, Floor G, et al. Strain development in unconfined coals exposed to CO2, CH4 and Ar: Effect of moisture. Int J Coal Geol, 2009, 77: 43–53
Majewska Z, Ceglarska-Stefanska G, Majewski S, et al. Binary gas sorption/desorption experiments on a bituminous coal: Simultaneous measurements on sorption kinetics, volumetric strain and acoustic emission. Int J Coal Geol, 2009, 77: 90–102
Larsen J W. The effects of dissolved CO2 on coal structure and properties. Int J Coal Geol, 2004, 57: 63–70
International Union of Pure and Applied Chemistry (IUPAC) Manual of Symbols and Terminology for Physico Chemical Quantities and Units. Butterworth: London, U.K., 1972
Dutta P, Harpalani S, Prusty B. Modeling of CO2 sorption on coal. Fuel, 2008, 87: 2023–2036
Harpalani S, Prusty B, Dutta P. Methane/CO2 sorption modeling for coalbed methane production and CO2 sequestration. Energy Fuels, 2006, 20: 1591–1599
Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc, 1918, 40: 1361–1403
Zhang T J, Xu H J, Li S G, et al. The effect of temperature on adsorbing capacity of coal (in Chinese). J Chin Coal Soc, 2009, 34: 802–805
Ruckenstein E, Vaidyanathan A S, Youngquist G R. Sorption by solids with bidisperse pore structures. Chem Eng Sci, 1971, 26: 1305–1318.
Clarkson C R, Bustin R M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 2. adsorption rate modeling. Fuel, 1999, 78: 1345–1362
Shi J Q, Durucan S. A bidisperse pore diffusion model for methane displacement desorption in coal by CO2 injection. Fuel, 2003, 82: 1219–1229
Cui X, Bustin R M, Dipple G. Selective transport of CO2, CH4, and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel, 2004, 83: 293–303
Karacan C O, Mitchell G D. Behavior and effect of different coal microlithotypes during gas transport for carbon dioxide sequestration into coal seams. Int J Coal Geol, 2003, 53: 201–217
Hildenbrand A, Krooss B M, Busch A, et al. Evolution of methane sorption capacity of coal seams as a function of burial history—A case study from the Campine Basin, NE Belgium. Int J Coal Geol, 2006, 66: 179–203
Radlinski A P, Busbridge T L, Gray E, et al. Small angle X-ray scattering mapping and kinetics study of sub-critical CO2 sorption by two Australian coals. Int J Coal Geol, 2009, 77: 80–89
Kelemen S R, Kwiatek L M. Physical properties of selected block argonne premium bituminous coal related to CO2, CH4, and N2 adsorption. Int J Coal Geol, 2009, 77: 2–9
Suuberg E M, Otake Y, Yun Y, et al. Role of moisture in coal structure and the effect of drying upon the accessibility of coal structure. Energy Fuels 1993, 7: 384–392
Thimons E D, Kissell F N. Diffusion of methane through coal. Fuel, 1973, 52: 274–280
Gruszkiewicz M S, Naney M T, Blencoe J G, et al. Adsorption kinetics of CO2, CH4, and their equimolar mixture on coal from the black warrior basin, west-central alabama. Int J Coal Geol, 2009, 77: 23–33
Siemons N, Wolf K, Bruining J. Interpretation of carbon dioxide diffusion behavior in coals. Int J Coal Geol, 2007, 72: 315–324
Hua F M. Prevention of coal and gas outbursts (in Chinese). Min Ind Sec Environ Prot, 2002, 29: 58–61
Stevens S H, Kuuskraa V A, Spector D, et al. CO2 sequestration in deep coal seams: Pilot results and worldwide potential. In: Riemer P, Eliasson B, Wokaun A, eds. Greenhouse Gas Control Technologies. Oxford: Elsevier, 1999. 175–180
Stevens S H, Spector D, Riemer P. Enhanced coalbed methane recovery using CO2 injection: Worldwide resource and CO2 sequestration potential. In: Proceedings of the International Oil & Gas Conference and Exhibition of the Society of Petroleum Engineers, 1998, November 2–6, Beijing, China, SPE Paper No. 48881, 489
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jian, X., Guan, P. & Zhang, W. Carbon dioxide sorption and diffusion in coals: Experimental investigation and modeling. Sci. China Earth Sci. 55, 633–643 (2012). https://doi.org/10.1007/s11430-011-4272-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11430-011-4272-4