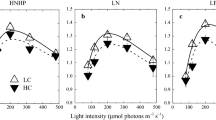
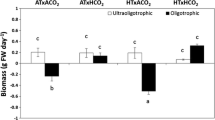
Abstract
Marine photosynthesis drives the oceanic biological CO2 pump to absorb CO2 from the atmosphere, which sinks more than one third of the industry-originated CO2 into the ocean. The increasing atmospheric CO2 and subsequent rise of pCO2 in seawater, which alters the carbonate system and related chemical reactions and results in lower pH and higher HCO3− concentration, affect photosynthetic CO2 fixation processes of phytoplanktonic and macroalgal species in direct and/or indirect ways. Although many unicellular and multicellular species can operate CO2-concentrating mechanisms (CCMs) to utilize the large HCO3− pool in seawater, enriched CO2 up to several times the present atmospheric level has been shown to enhance photosynthesis and growth of both phytoplanktonic and macro-species that have less capacity of CCMs. Even for species that operate active CCMs and those whose photosynthesis is not limited by CO2 in seawater, increased CO2 levels can down-regulate their CCMs and therefore enhance their growth under light-limiting conditions (at higher CO2 levels, less light energy is required to drive CCM). Altered physiological performances under high-CO2 conditions may cause genetic alteration in view of adaptation over long time scale. Marine algae may adapt to a high CO2 oceanic environment so that the evolved communities in future are likely to be genetically different from the contemporary communities. However, most of the previous studies have been carried out under indoor conditions without considering the acidifying effects on seawater by increased CO2 and other interacting environmental factors, and little has been documented so far to explain how physiology of marine primary producers performs in a high-CO2 and low-pH ocean.
Similar content being viewed by others
References
Bowes G. Facing the inevitable plants and increasing atmospheric CO2. Annu Rev Plant Physiol Plant Mol Biol, 1993, 44: 309–332, 10.1146/annurev.pp.44.060193.001521, 1:CAS:528:DyaK3sXlsFKisbs%3D
Ziska L H, Bunce J A. Plant responses to rising atmospheric carbon dioxide. In: Morison J I L, Morecroft M D, eds. Plant Growth and Climate Change. Oxford: Blackwell Publishers, 2006, 17–45, 10.1002/9780470988695.ch2
Zhang Q, Lu C, Kuang T. Effects of the rising CO2 levels on photosynthesis. Chin Bull Bot, 1992, 9: 18–23
Ainsworth E A, Long S P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A metaanalytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol, 2005, 165: 351–372, 15720649, 10.1111/j.1469-8137.2004.01224.x
Gao K, McKinley K R. Use of macroalgae for marine biomass production and CO2 remediation: a review. J Appl Phycol, 1994, 6:45–60, 10.1007/BF02185904
Badger M R, Andrews T J, Whitney S M, et al. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot, 1998, 76:1052–1071, 10.1139/cjb-76-6-1052, 1:CAS:528:DyaK1cXotVeksbY%3D
Caldeira K, Wickett M E. Oceanography: anthropogenic carbon and ocean pH. Nature, 2003, 425: 365, 14508477, 10.1038/425365a, 1:CAS:528:DC%2BD3sXnsV2ktrs%3D
Feely R A, Sabine C L, Lee K, et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science, 2004, 305: 362–366, 15256664, 10.1126/science.1097329, 1:CAS:528:DC%2BD2cXls1egsbY%3D
Sabine C L, Feely R A, Gruber N, et al. The oceanic sink for anthropogenic CO2. Science, 2004, 305: 367–371, 15256665, 10.1126/science.1097403, 1:CAS:528:DC%2BD2cXls1egsbc%3D
Behrenfeld M J, O’Malley R T, Siegel D A, et al. Climate-driven trends in contemporary ocean productivity, Nature, 2006, 444: 752–755, 17151666, 10.1038/nature05317, 1:CAS:528:DC%2BD28Xht1Ontb3M
Quay P D, Tilbrook B, Wong C S. Oceanic uptake of fossil fuel CO2: carbon-13 evidence. Science, 1992, 256: 74–79, 17802595, 10.1126/science.256.5053.74, 1:CAS:528:DyaK38Xit1SmtLo%3D
Murray J W, Barber R T, Roman M R, et al. Physical and biological controls on carbon cycling in equatorial pacific. Science, 1994, 266: 58–65, 17814000, 10.1126/science.266.5182.58, 1:CAS:528:DyaK2cXmtlOrt7c%3D
Arrigo K R. Carbon cycle: Marine manipulations. Nature, 2007, 450:491–492, 18033286, 10.1038/450491a, 1:CAS:528:DC%2BD2sXhtlCrtbjJ
Raven J A. Limits on growth rates. Nature, 1993, 361: 209–210, 10.1038/361209a0
Riebesell U, Wolf-Gladrow D A, Smetacek V S. Carbon dioxide limitation of marine phytoplankton growth rates. Nature, 1993, 361: 249–251, 10.1038/361249a0, 1:CAS:528:DyaK3sXhs1Cqurc%3D
Morel F M M, Reinfelder J R, Roberts S B, et al. Zinc and carbon co-limitation of marine phytoplankton. Nature, 1994, 369: 740 742, 10.1038/369740a0
Hein M, Sand-Jensen K. CO2 increases oceanic primary production. Nature, 1997, 388: 526–527, 10.1038/41457, 1:CAS:528:DyaK2sXlt1arsbk%3D
Schippers P, Lürling M. Increase of atmospheric CO2 promotes phytoplankton productivity. Ecol Lett, 2004, 7: 446–451, 10.1111/j.1461-0248.2004.00597.x
Beardall J, Raven J A. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia, 2004, 43: 26–40
Giordano M, Beardall J, Raven J A. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation and evolution. Annu Rev Plant Biol, 2005, 56: 99–131, 15862091, 10.1146/annurev.arplant.56.032604.144052, 1:CAS:528:DC%2BD2MXmtVaru7w%3D
Kaplan A, Badger M R, Berry J A. Photosynthesis and the intracellular inorganic pool in the blue-green alga Anabaena variabilis: Response to external CO2 concentration. Planta, 1980, 149: 219–226, 10.1007/BF00384557, 1:CAS:528:DyaL3cXlsVKmt74%3D
Badger M R, Gallagher A. Adaptation of photosynthetic CO2 and HCO3-accumulation by the cyanobacterium synechococcus PCC6301 to growth at different inorganic carbon concentrations. Aust J Plant Physiol, 1987, 14: 189–201, 1:CAS:528:DyaL2sXkslequrY%3D
Tsuzuki M, Miyachi S. The function of carbonic anhydrase in aquatic photosynthesis. Aquat Bot, 1989, 34: 85–104, 10.1016/0304-3770(89)90051-X, 1:CAS:528:DyaL1MXltlSltLg%3D
Raven J A. Physiology of inorganic C acquisition and implications for resource use efficiency by marine phytoplankton relation to increased CO2 and temperature. Plant Cell Environ, 1991, 14: 779–794, 10.1111/j.1365-3040.1991.tb01442.x, 1:CAS:528:DyaK38XhsVyisbc%3D
Raven J A. Photosynthetic and non-photosynthetic roles of carbonic anhydrase in algae and cyanobacteria. Phycologia, 1995, 34: 93–101
Rost B, Riebesell U, Burkhardt S, et al. Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr, 2003, 48: 55–67
Yang Y, Gao K. Effects of CO2 concentrations on the freshwater microalgae, Chlamydomonas reinhardtii, Chlorella pyrenoidosa and Scenedesmus obliquus (Chlorophyta). J Appl Phycol, 2003, 15: 1–11, 10.1023/A:1026021021774
Qiu B, Gao K. Effects of CO2 enrichment on the bloom-forming cyanobacterium Microcystis aeruginosa (Cyanophyceae): physiological responses and relationships with the availability of dissolved inorganic carbon. J Phycol, 2002, 38: 721–729, 10.1046/j.1529-8817.2002.01180.x, 1:CAS:528:DC%2BD38XnsVCqtrs%3D
Riebesell U, Wolf-Gladrow D A, Smetacek V S. Carbon dioxide limitation of marine phytoplankton growth rates. Nature, 1993,361: 249–251, 10.1038/361249a0, 1:CAS:528:DyaK3sXhs1Cqurc%3D
Chen X, Gao K. Photosynthetic utilisation of inorganic carbon and its regulation in the marine diatom Skeletonema costatum. Funct Plant Biol, 2004, 31:1027–1033, 10.1071/FP04076, 1:CAS:528:DC%2BD2cXosFSqu7Y%3D
Collins S, Sültemeyer D, Bell G. Changes in C uptake in populations of Chlamydomonas reinhardtii selected at high CO2. Plant Cell Environ, 2006, 29: 1812–1819, 16913870, 10.1111/j.1365-3040.2006.01559.x, 1:CAS:528:DC%2BD28XhtVWktLnF
Riebesell U, Zondervan I, Rost B, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature, 2000, 407: 364–367, 11014189, 10.1038/35030078, 1:CAS:528:DC%2BD3cXntFyrs7o%3D
Xia J R, Gao K S. Impacts of elevated CO2 concentration on biochemical composition, carbonic anhydrase, and nitrate reductase activity of freshwater green algae. J Integr Plant Biol, 2005, 47: 668–675, 1:CAS:528:DC%2BD28Xpt1yqsw%3D%3D, 10.1111/j.1744-7909.2005.00114.x
Chen X, Gao K. Effect of CO2 concentrations on the activity of photosynthetic CO2 fixation and extracellular carbonic anhydrase in the marine diatom Skeletonema costatum. Chin Sci Bull, 2003, 48, 2616–2620, 10.1360/03wc0084, 1:CAS:528:DC%2BD2cXmslyktA%3D%3D
Burkhardt S, Amoroso G, Riebesell U, et al. CO2 and HCO3− uptake in marine diatoms acclimated to different CO2 concentrations. Limnol Oceanogr, 2001, 46: 1378–1391, 1:CAS:528:DC%2BD3MXnsVSktbs%3D
Burkhardt S, Riebesell U. CO2 availability affects elemental composition (C: N: P) of the marine diatom Skeletonema costatum. Mar Ecol Prog Ser, 1997, 155: 67–76, 10.3354/meps155067, 1:CAS:528:DyaK2sXmt12hsb4%3D
Burkhardt S, Zondervan I, Riebesell U. Effect of CO2 concentration on C: N: P ratio in marine phytoplankton: A species comparison. Limnol Oceanogr, 1999, 44: 683–690, 1:CAS:528:DyaK1MXjs1yjs7g%3D
Andersen T, Andersen F Ø. Effects of CO2 concentration on growth of filamentous algae and Littorella uniflora in a Danish Softwater lake. Aquat Bot, 2006, 84: 267–271, 10.1016/j.aquabot.2005.09.009, 1:CAS:528:DC%2BD28XhsF2gtbs%3D
Qiu B S, Liu J Y. Utilization of inorganic carbon in the edible cyanobacterium Ge-Xian-Mi (Nostoc) and its role in alleviating photo-inhibition. Plant Cell Environ, 2004, 27, 1447–1458, 10.1111/j.1365-3040.2004.01248.x, 1:CAS:528:DC%2BD2MXjvFOrtg%3D%3D
Reiskind J B, Beer S, Bowes G Photosynthesis, photorespiration and ecophysiological interactions in marine macroalgae. Aquat Bot, 1989, 34: 131–152, 10.1016/0304-3770(89)90053-3, 1:CAS:528:DyaL1MXltlSltLY%3D
Beer S. Mechanisms of inorganic carbon acquisition in marine maroalgae (with reference to the Chlorophyta). Prog Phycol Res, 1994, 10: 179–207, 1:CAS:528:DyaK2MXlvFGhsrw%3D
Beer S, Koch E. Photosynthesis of seagrasses and marine macroalgae in globally changing CO2 environments. Mar Ecol Prog Ser, 1996, 141: 199–204, 10.3354/meps141199
Raven J A. Inorganic carbon acquisition by marine autotrophs. Adv Bot Res, 1997, 27: 85–209, 10.1016/S0065-2296(08)60281-5, 1:CAS:528:DyaK2sXnt12msr8%3D
Larsson C, Axelsson L. Bicarbonate uptake and utilization in marine macroalgae. Eur J Phycol, 1999, 34: 79–86, 10.1080/09670269910001736112
Drechsler Z, Sharkia R, Cabantchik Z, et al. Bicarbonate uptake in the marine maxroalga Ulva sp. is inhibited by classical probes of anion exchange by red blood cells. Planta, 1993, 191: 34–40, 10.1007/BF00240893, 1:CAS:528:DyaK3sXlsFGqsrk%3D
Drechsler Z, Sharkia R, Cabantchik Z I, et al. The relationship of arginine groups to photosynthetic HCO3− uptake in Ulva sp. mediated by a putative anion exchanger. Planta, 1994, 194: 250–255, 10.1007/BF01101685, 1:CAS:528:DyaK2cXksFCitbY%3D
Axelsson L, Ryberg H, Beer S. Two modes of bicarbonate utilization in the marine green macroalga Ulva lactuca. Plant Cell Environ, 1995, 18: 439–445, 10.1111/j.1365-3040.1995.tb00378.x, 1:CAS:528:DyaK2MXlslCktr4%3D
Gao K, Aruga Y, Asada K, et al. Enhanced growth of the red alga Porphyra yezoensis Ueda in high CO2 concentrations. J Appl Phycol, 1991, 3: 356–362
Gao K, Aruga Y, Asada K, et al. Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis. J Appl Phycol, 1993, 5: 563–571, 10.1007/BF02184635, 1:CAS:528:DyaK2cXjtF2isbk%3D
Zou D H, Gao K S. Ecophysiological characteristics of four intertidal marine macroalgae during emersion along Shantou Coast of China, with a special reference to the relationship of photosynthesis and CO2. Acta Ocean Sin, 2005, 24(3): 105–113
Kübler J E, Johnston A M, Raven J A. The effects reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata. Plant Cell Environ, 1999, 22: 1303–1310, 10.1046/j.1365-3040.1999.00492.x
Björk M, Haglund K, Ramazanov Z, et al. Inducible mechanism for HCO3− utilization and repression of photorespiration in protoplasts and thallus of three species of Ulva (Chlorophyta). J Phycol, 1993, 29: 166–173, 10.1111/j.0022-3646.1993.00166.x
Mercado J M, Gordillo F J L, Figueroa F L, et al. External carbonic anhydrase and affinity for inorganic carbon in intertidal macroalgae. J Exp Mar Biol Ecol, 1998, 221: 209–220, 10.1016/S0022-0981(97)00127-5, 1:CAS:528:DyaK1cXhvVSnur4%3D
Gordillo F J L, Niell F X, Figueroa F L. Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta, 2001, 213: 64–70, 11523657, 10.1007/s004250000468, 1:CAS:528:DC%2BD3MXjt1Chs7Y%3D
García-Sânchez M J, Fernândez J A, Niell F X. Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta, 1994, 194: 55–61, 10.1007/BF00201034
Mercado J M, Javier F, Gordillo L, et al. Effects of different leverls of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J Appl Phycol, 1999, 11: 455–461, 10.1023/A:1008194223558
Israel A, Katz S, Dubinsky Z. et al. Photosynthetic inorganic carbon utilization and growth of Porphyra linearis (Rhorophyta). J Appl Phycol, 1999, 11: 447–453, 10.1023/A:1008122306268
Israel A, Hophy M. Growth, photosynthetic properties and Rubisco activies and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Glob Change Biol, 2002, 8: 831–840, 10.1046/j.1365-2486.2002.00518.x
Zou D, Gao K S, Ruan Z X. Effects of elevated CO2 concentration on photosynthesis and nutrients uptake of Ulva lactuca. J Ocean Univ Qingdao, 2001, 31: 877–882, 1:CAS:528:DC%2BD38XislWrsw%3D%3D
Andria J R, Vergara J J, Perez-Llorens J L. Biochemical responses and photosynthetic performance of Gracilaria sp. (Rhodophyta) from Cadiz, Spain, cultured under different inorganic carbon and nitrogen levels. Eur J Phycol, 1999, 34: 497–504, 10.1080/09541449910001718851
Gao K, Aruga Y, Asada K, et al. Calcification in the articulated coralline alga Corallina pilulifera, with special reference to the effect of elevated CO2 concentration. Mar Biol, 1993, 117: 129–132, 10.1007/BF00346434, 1:CAS:528:DyaK2cXkt1yi
Langdon C, Broecker W S, Hammond D E, et al. Effect of elevated CO2 on the community metabolism of an experimental coral reef. Global Biogeocheml Cy, 2003, 17(1): 1–14
Maberly S C, Madsen T V. Contribution of air and water to the carbon balance of Fucus spiralis. Mar Ecol Prog Ser, 1990, 62: 175–183, 10.3354/meps062175
Peňa E J, Zingmark R, Nietch C. Comparative photosynthesis of two species of intertidal epiphytic macroalgae on mangrove roots during submersion and emersion. J Phycol, 1999, 35: 1206–1214, 10.1046/j.1529-8817.1999.3561206.x
Mercado J M, Niell F X. Carbon dioxide uptake by Bostrychia scorpioides (Rhodophyceae) under emersed conditions. Eur J Phycol, 2000, 35: 45–51, 10.1080/09670260010001735611
Zou D H, Gao K S. Exogenous carbon acquisition of photosynthesis in Porphyra haitanensis (Bangiales, Rhodophyta) under emersed state. Prog Nat Sci, 2004,14(2): 34–40, 10.1080/10020070412331343271
Gao K, Ji Y, Aruga Y. Relationship of CO2 concentrations to photosynthesis of intertidal macrioalgae during emersion. Hydrobiologia, 1999, 398/399: 355–359, 10.1023/A:1017072303189
Zou D H, Gao K S. Effects of desiccation and CO2 concentrations on emersed photosynthesis in Porphyra haitanensis (Bangiales, Rhodophyta), a species farmed in China. Eur J Phycol, 2002, 37: 587–592, 10.1017/S0967026202003876
Zou D H, Gao K S, Run Z X. Daily timing of emersion and elevated atmospheric CO2 concentration affect photosynthetic performance of the intertidal macroalga Ulva lactuca (Chorophyta) in sunlight. Bot Mar, 2007, 50: 275–279, 10.1515/BOT.2007.031, 1:CAS:528:DC%2BD1cXhtFOjsro%3D
Zou D H. Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture, 2005, 250: 726–735, 10.1016/j.aquaculture.2005.05.014, 1:CAS:528:DC%2BD2MXht1OrsLnN
Gao K, Guan W, Helbling E W. Effects of solar ultraviolet radiation on photosynthesis of the marine red tide alga Heterosigma akashiwo (Raphidophyceae). J Photoch Photobio B, 2007, 86: 140–148, 10.1016/j.jphotobiol.2006.05.007, 1:CAS:528:DC%2BD2sXhsFentw%3D%3D
Gao K, Wu Y P, Li G, et al. Solar UV-radiation drives CO2-fixation in marine phytoplankton: A double-edged sword. Plant Physiol, 2007, 144: 54–59, 17494919, 10.1104/pp.107.098491, 1:CAS:528:DC%2BD2sXls1Kjsbo%3D
Wu H Y, Gao K, Villafañe V E, et al. Effects of solar UV radiation on morphology and photosynthesis of the filamentous cyanobacterium, Arthrospira platensis. Appl Environ Microb, 2005, 71(9): 5004–5013, 10.1128/AEM.71.9.5004-5013.2005, 1:CAS:528:DC%2BD2MXhtVahsbnN
Gao K, Li P, Watanabe T, et al. Combined effects of ultraviolet radiation and temperature on morphology, photosynthesis and DNA of Arthrospira (Spirulina) platensis (Cyanophyta). J Phycol, 2008, 44: 777–786, 10.1111/j.1529-8817.2008.00512.x
Riebesell U, Schulz K G, Bellerby R G J, et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature, 2007, 450: 544–549, 10.1038/nature06267, 1:CAS:528:DC%2BD2sXhtlCrtbrI
Collins S, Bell G. Phenotypic consequences of 1000 generations of selection at elevated CO2 in a green alga. Nature, 2004, 431: 566–569, 15457260, 10.1038/nature02945, 1:CAS:528:DC%2BD2cXnvFCnurk%3D
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Natural Science Foundation of China (Grant Nos. 90411018, and 40676063), National Basic Research Program of China (Grant No. 2009CB 421207) and Ministry of Education of China for Key Profect (Grant No. 308015)
Rights and permissions
About this article
Cite this article
Wu, H., Zou, D. & Gao, K. Impacts of increased atmospheric CO2 concentration on photosynthesis and growth of micro- and macro-algae. SCI CHINA SER C 51, 1144–1150 (2008). https://doi.org/10.1007/s11427-008-0142-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-008-0142-5