Abstract
Purpose
Most previous studies focused on the metal extraction efficiency of chelant-enhanced soil washing under various operational conditions; however, one of the keys to cost-effective field application is to maintain a high throughput rate (i.e., short washing time) while achieving the treatment objectives. Therefore, this study presented a conceptual model for simulating the kinetic extraction of heavy metals and evaluated its sensitivity analysis to the predetermined parameter values in five soils with different initial metal distributions.
Materials and methods
The proposed conceptual model considered the initial metal distribution and the respective fast and slow first-order extraction rates as the predetermined input parameters. The sensitivity analysis was conducted by combined and individual variation of the parameter values by fixed percentages. An implicit assumption is that there is no interaction between the metal fractions and the parameters are independent of each other. All other parameters were kept constant. Such systematic testing of the model behaviour in reaction to changes in the input parameters helped us identify the parameters that are more sensitive and require particular attention in the modelling process.
Results and discussion
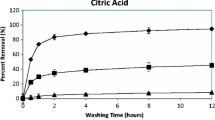
In the sensitivity analysis of combined parameter groups, the fraction of fast extraction (f) was linearly and inversely correlated to the required washing time in two stages, in which the correlation was more significant at the lower range of f values. The first-order rate coefficients of fast extraction (k′) and slow extraction (k″) showed a nonlinear and negative relationship with the required washing time. The individual contributions of f i and k i′ of the three fraction groups were then analysed. Although the fraction group 1 (exchangeable and carbonate) had the highest f i and k i′ values, their importance was limited by relatively small proportion in the field-contaminated soils. The required washing time for Pb extraction was most sensitive to the fraction group 2 (Fe/Mn oxides).
Conclusions
The results of this study suggested that model simulations are less sensitive to f 1 and k 1′ values of exchangeable and carbonate fractions, which can therefore be considered constant. However, f 2 and k 2′ values of the oxide fraction are sensitive parameters and should be prudently justified, or even recalibrated, at different sites.








Similar content being viewed by others
References
Anderson R, Rasor E, Van Ryn F (1999) Particle size separation via soil washing to obtain volume reduction. J Hazard Mater 66:89–98
Barona A, Aranguiz I, Elías A (2001) Metal associations in soils before and after EDTA extractive decontamination: implications for the effectiveness of further clean-up procedures. Environ Pollut 113:79–85
Bermond A, Ghestem JP (2001) Kinetic study of trace metal EDTA-desorption from contaminated soils. In: Selim HM, Sparks DL (eds) Heavy metals release in soils. Lewis, Boca Raton, pp 131–147
Council of the European Union (1999) Council Directive 1999/31/EC of 26 April 1999 on the landfill of waste
Dermont G, Bergeron M, Mercier G, Richer-Lafleche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152:1–31
Di Palma L, Mecozzi R (2007) Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents. J Hazard Mater 147:768–775
Elless MP, Blaylock MJ (2000) Amendment optimization to enhance lead extractability from contaminated soils for phytoremediation. Int J Phytoremediation 2:75–89
Fangueiro D, Bermond A, Santos E, Carapuça H, Duarte A (2002) Heavy metal mobility assessment in sediments based on a kinetic approach of the EDTA extraction: Search for optimal experimental conditions. Anal Chim Acta 459:245–256
Fangueiro D, Bermond A, Santos E, Carapuça H, Duarte A (2005) Kinetic approach to heavy metal mobilization assessment in sediments: choose of kinetic equations and models to achieve maximum information. Talanta 66:844–857
Finzgar N, Lestan D (2006) Heap leaching of Pb and Zn contaminated soil using ozone/UV treatment of EDTA extractants. Chemosphere 63:1736–1743
Friedly JC, Kent DB, Davis JA (2002) Simulation of the mobility of metal-EDTA complexes in groundwater: the influence of contaminant metals. Environ Sci Technol 36:355–363
Griffiths RA (1995) Soil-washing technology and practice. J Hazard Mater 40:175–189
Harbottle MJ, Al-Tabbaa A, Evans CW (2007) A comparison of the technical sustainability of in situ stabilisation/solidification with disposal to landfill. J Hazard Mater 141:430–440
Jalali M, Khanlari ZV (2008) Effect of aging process on the fractionation of heavy metals in some calcareous soils of Iran. Geoderma 143:26–40
Kent DB, Davis JA, Joye JL, Curtis GP (2008) Influence of variable chemical conditions on EDTA-enhanced transport of metal ions in mildly acidic groundwater. Environ Pollut 153:44–52
Kirpichtchikova TA, Manceau A, Spadini L, Panfili F, Marcus MA, Jacquet T (2006) Speciation and solubility of heavy metals in contaminated soil using X-ray microfluorescence, EXAFS spectroscopy, chemical extraction, and thermodynamic modeling. Geochim Cosmochim Acta 70:2163–2190
Koopmans GF, Schenkeveld WDC, Song J, Luo Y, Japenga J, Temminghoff EJM (2008) Influence of EDDS on metal speciation in soil extracts: measurement and mechanistic multicomponent modeling. Environ Sci Technol 42:1123–1130
Mann MJ (1999) Full-scale and pilot-scale soil washing. J Hazard Mater 66:119–136
Meers E, Tack FMG, Verloo MG (2008) Degradability of ethylenediaminedisuccinic acid (EDDS) in metal contaminated soils: implications for its use soil remediation. Chemosphere 70:358–363
Nowack B (2002) Environmental chemistry of aminopolycarboxylate chelating agents. Environ Sci Technol 36:4009–4016
Nowack B, VanBriesen JM (2005) Biogeochemistry of chelating agents. American Chemical Society, Washington, DC
Nowack B, Schulin R, Robinson BH (2006) Critical assessment of chelant-enhanced metal phytoextraction. Environ Sci Technol 40:5225–5232
Peters RW (1999) Chelant extraction of heavy metals from contaminated soils. J Hazard Mater 66:151–210
Polettini A, Pomi R, Rolle E, Ceremigna D, De Propris L, Gabellini M, Tornato A (2006) A kinetic study of chelant-assisted remediation of contaminated dredged sediment. J Hazard Mater 137:1458–1465
Polettini A, Pomi R, Rolle E (2007) The effect of operating variables on chelant-assisted remediation of contaminated dredged sediment. Chemosphere 66:866–877
Pronk JP (2000) Circular on target values and intervention values for soil remediation. Hague
Tandy S, Bossart K, Mueller R, Ritschel J, Hauser L, Schulin R, Nowack B (2004) Extraction of heavy metals from soils using biodegradable chelating agents. Environ Sci Technol 38:937–944
Tandy S, Ammann A, Schulin R, Nowack B (2006) Biodegradation and speciation of residual SS-ethylenediaminedisuccinic acid (EDDS) in soil solution left after soil washing. Environ Pollut 142:191–199
Trivedi P, Dyer JA, Sparks DL (2003) Lead sorption onto ferrihydrite. 1. A macroscopic and spectroscopic assessment. Environ Sci Technol 37:908–914
Tsang DCW, Lo IMC (2006) Competitive Cu and Cd sorption and transport in soils: a combined batch kinetics, column, and sequential extraction study. Environ Sci Technol 40:6655–6661
Tsang DCW, Lo IMC, Chan KL (2007a) Modeling the transport of metals with rate-limited EDTA-promoted extraction and dissolution during EDTA-flushing of copper-contaminated soils. Environ Sci Technol 41:3660–3666
Tsang DCW, Zhang W, Lo IMC (2007b) Copper extraction effectiveness and soil dissolution issues of EDTA-flushing of artificially contaminated soils. Chemosphere 68:234–243
Tsang DCW, Zhang W, Lo IMC (2007c) Modeling cadmium transport in soils using sequential extraction, batch, and miscible displacement experiments. Soil Sci Soc Am J 71:674–681
Tsang DCW, Yip TCM, Lo IMC (2009) Kinetic interactions of EDDS with soils. 2. Metal-EDDS complexes in uncontaminated and metal-contaminated soils. Environ Sci Technol 43:837–842
U.S. Department of Defense (1997) ESTCP Cost and performance report: joint small-arms range remediation. Environmental Security Technology Certification program (ESTCP), U.S. Department of Defense, Arlington
Udovic M, Lestan D (2009) Pb, Zn and Cd mobility, availability and fractionation in aged soil remediated by EDTA leaching. Chemosphere 74:1367–1373
van Hees PAW, Elgh-Dalgren K, Engwall M, von Kronhelm T (2008) Re-cycling of remediated soil in Sweden: an environmental advantage? Resour Conserv Recycl 52:1349–1361
van Waveren RH, Groot S, Scholten H, van Geer F, Wosten H, Koeze R, Noort J (2000) Good modelling practice handbook. STOWA, Utrecht
Vandevivere P, Hammes F, Verstraete W, Feijtel T, Schowanek D (2001) Metal decontamination of soil, sediment, and sewage sludge by means of transition metal chelant [S, S]-EDDS. J Environ Eng 127:802–811
Wasay SA, Barrington S, Tokunagal S, Prasher S (2007) Kinetics of heavy metal desorption from three soils using citric acid, tartaric acid, and EDTA. J Environ Eng Sci 6:611–622
Whitworth DJ, Achterberg EP, Herzl V, Nimmo M, Gledhill M, Worsfold PJ (1999) Development of a simple extraction procedure using ligand competition for biogeochemically available metals of estuarine suspended particulate matter. Anal Chim Acta 392:3–17
Yan DYS, Yui MMT, Yip TCM, Tsang DCW, Lo IMC (2010) Influence of EDDS-to-metal molar ratio, solution pH, and soil-to-solution ratio on metal extraction under EDDS deficiency. J Hazard Mater 178:890–894
Yip TCM, Tsang DCW, Ng KTW, Lo IMC (2009a) Kinetic interactions of EDDS with soils. 1. Metal resorption and competition under EDDS deficiency. Environ Sci Technol 43:831–836
Yip TCM, Tsang DCW, Ng KTW, Lo IMC (2009b) Empirical modeling of heavy metal extraction by EDDS from single-metal and multi-metal contaminated soils. Chemosphere 74:301–307
Yip TCM, Tsang DCW, Lo IMC (2010a) Interactions of chelating agents with Pb-goethite at the solid-liquid interface: Pb extraction and re-adsorption. Chemosphere 81:415–421
Yip TCM, Yan DYS, Yui MMT, Tsang DCW, Lo IMC (2010b) Heavy metal extraction from an artificially contaminated sandy soil under EDDS deficiency: significance of humic acid and chelant mixture. Chemosphere 80:416–421
Yu J, Klarup D (1994) Extraction kinetics of copper, zinc, iron, and manganese from contaminated sediment using disodium ethylenediaminetetraacetate. Water Air Soil Pollut 75:205–225
Zhang W, Tsang DCW, Lo IMC (2007) Removal of Pb and MDF from contaminated soils by EDTA- and SDS-enhanced washing. Chemosphere 66:2025–2034
Zhang W, Tsang DCW, Lo IMC (2008) Removal of Pb by EDTA-washing in the presence of hydrophobic organic contaminants or anionic surfactant. J Hazard Mater 155:433–439
Zhang W, Huang H, Tan F, Wang H, Qiu R (2010) Influence of EDTA washing on the species and mobility of heavy metals residual in soils. J Hazard Mater 173:369–376
Zou Z, Qiu R, Zhang W, Dong H, Zhao Z, Zhang T, Wei X, Cai X (2009) The study of operating variables in soil washing with EDTA. Environ Pollut 157:229–236
Acknowledgements
The authors wish to thank the Research Grants Council of Hong Kong for providing financial support under the General Research Fund (Project account 616608) for this research study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Qixing Zhou
Rights and permissions
About this article
Cite this article
Tsang, D.C.W., Yip, T.C.M. & Lo, I.M.C. Conceptual model and sensitivity analysis for simulating the extraction kinetics of soil washing. J Soils Sediments 11, 1221–1233 (2011). https://doi.org/10.1007/s11368-011-0400-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0400-1