Abstract
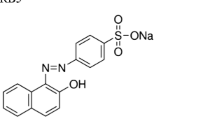
In this study, the white-rot fungus Cyathus bulleri was cultivated on low-cost agro-residues, namely wheat bran (WB), wheat straw (WS), and domestic waste orange peel (OP) for production of ligninolytic enzymes. Of the three substrates, WB and OP served as good materials for the production of laccase with no requirement of additional carbon or nitrogen source. Specific laccase activity of 94.4 U mg−1 extracellular protein and 21.01 U mg−1 protein was obtained on WB and OP, respectively. Maximum decolorization rate of 13.6 μmol h−1 U−1 laccase for reactive black 5 and 22.68 μmol h−1 U−1 laccase for reactive orange 16 (RO) was obtained with the WB culture filtrate, and 11.7 μmol h−1 U−1 laccase for reactive violet 5 was observed with OP culture filtrate. Importantly, Kiton blue A (KB), reported not to be amenable to enzymatic degradation, was degraded by culture filtrate borne activities. Products of degradation of KB and RO were identified by mass spectrometry, and a pathway of degradation proposed. WB-grown culture filtrate decolorized and detoxified real and simulated textile effluents by about 40%. The study highlights the use of inexpensive materials for the production of enzymes effective on dyes and effluents.







Similar content being viewed by others
References
Adak A, Tiwari R, Singh S, Sharma S, Nain L (2016) Laccase production by a novel white-rot fungus Pseudolagarobasidium acaciicola LA 1 through solid-state fermentation of Parthenium biomass and its application in dyes decolorization. Waste Biomass Valor 7:1–9
Anastasi A, Parato B, Spina F, Tigini V, Prigione V, Varese GC (2011) Decolourisation and detoxification in the fungal treatment of textile wastewaters from dyeing processes. New Biotechnol 29:38–45
APHA (2005) Standard methods for examination of water and wastewater, American Public Health Association WWA. Washington, D.C.
Bashir H, Gangwar R, Mishra S (2015) Differential production of lignocellulolytic enzymes by a white rot fungus Termitomyces sp. OE147 on cellulose and lactose. BiochimBiophys Acta 1854:1290–1299
Bedekar PA, Saratale RG, Saratale GD, Govindwar SP (2014) Oxidative stress response in dye degrading bacterium Lysinibacillus sp. RGS exposed to reactive orange 16, degradation of RO16 and evaluation of toxicity. Environ Sci Pollut Res 21:11075–11085
Bheemaraddi MC, Patil S, Shivannavar CT, Gaddad SM (2014) Isolation and characterization of Paracoccus sp. GSM2 capable of degrading textile azo dye reactive violet 5. Sci World J 410704.
Bumpus JA, Brock BJ (1988) Biodegradation of crystal violet by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol 54:1143–1150
Camarero S (1999) Description of a versatile peroxidase involved in the natural degradation of lignin that has both manganese peroxidase and lignin peroxidase substrate interaction sites. J Biol Chem 274:10324–10330
Camarero S, Ibarra D, Martinez AT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71:1775–1784
Cesaro A, Naddeo V, Belgiorno V (2013) Wastewater treatment by combination of advanced oxidation processes and conventional biological systems. J Bioremed Biodeg 4:208
Chen CY (2009) Photocatalytic degradation of azo dye reactive orange 16 by TiO2. Water Air Soil Pollut 202:335–342
Chhabra M, Mishra S, Sreekrishnan TR (2008) Mediator-assisted decolorization and detoxification of textile dyes/dye mixture by Cyathus bulleri laccase. Appl Biochem Biotechnol 151:587–598
Chhabra M, Mishra S, Sreekrishnan TR (2009) Laccase/mediator assisted degradation of triarylmethane dyes in a continuous membrane reactor. J Biotechnol 143:69–78
Couto SR (2009) Dye removal by immobilized fungi. Biotechnol Adv 27:227–235
D’Souza TM, Merritt CS, Reddy CA (1999) Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Appl Environ Microbiol 65:5307–5313
Dhillon GS, Kaur S, Brar SK (2012) In-vitro decolorization of recalcitrant dyes through an eco-friendly approach using laccase from Trametes versicolor grown on brewer’s spent grain. Int Biodeter Biodegr 72:67–75
Eggert C, Temp U, Eriksson KEL (1996) The ligninolytic system of white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Elisashvili V, Penninckx M, Kachlishvili E, Tsiklauri N, Metreveli E, Khaziani T, Kvesitadze G (2008) Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition. Bioresour Technol 99:457–462
Farnet AM, Criquet S, Cigna M, Gil G, Ferre E (2004) Purification of a laccase from Marasmius quercophilus induced with ferulic acid: reactivity towards natural and xenobiotic aromatic compounds. Enzym Microb Technol 34:549–554
Fragner D, Zomorrodi M, Kues U, Majcherczyk A (2009) Optimized protocol for the 2-DE of extracellular proteins from higher basidiomycetes inhabiting lignocellulose. Electrophoresis 30:2431–2441
Franciscon E, Grossman MJ,Paschoal JAR,Reyes FGR,Durrant LR (2012) Decolorization and biodegradation of reactive sulfonated azo dyes by a newly isolated Brevibacterium sp. strain VN-15. Springer Plus 1–37.
Hao OJ, Kim H, Chiang PC (2000) Decolorization of waste water. Crit Rev Env Sci 30:449–505
Harms H, Schlosser D, Wick LY (2011) Untapped potential: exploiting fungi in bioremediation of hazardous chemicals. Nat Rev Microbiol 9:177–192
Hegde S, Kavitha S, Varadaraj MC, Muralikrishna G (2006) Degradation of cereal bran polysaccharide-phenolic acid complexes by Aspergillus niger CFR 1105. Food Chem 96:14–19
Karp SG, Faraco V, Amore A, Birolo L, Giangrande C, Soccol VT, Pandey A, Soccol CR (2012) Characterization of laccase isoforms produced by Pleurotus ostreatus in solid state fermentation of sugarcane bagasse. Bioresour Technol 114:735–739
Kenzom T, Srivastava P, Mishra S (2014) Structural insights into 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)-mediated degradation of reactive blue 21 by engineered Cyathus bulleri laccase and characterization of degradation products. Appl Environ Microbiol 80:7484–7495
Lee JW, Choi SP, Thiruvenkatachari R, Shim WG, Moon H (2006) Submerged microfiltration membrane coupled with alum coagulation/powdered activated carbon adsorption for complete decolorization of reactive dyes. Water Res 40:435–444
Majeau JA, Brar SK, Tyagi RD (2010) Laccases for removal of recalcitrant and emerging pollutants. Bioresour Technol 101:2331–2350
Malarvizhi K, Murugesan K, Kalaichelvan PT (2003) Xylanase production by Ganoderma lucidum on liquid and solid state culture. Indian J Exp Biol 41:620–626
Margot J, Maillard J, Rossi L, Barry DA, Holliger C (2013) Influence of treatment conditions on the oxidation of micropollutants by Trametes versicolor laccase. New Biotechnol 30:803–813
Mate DM, Alcalde M (2015) Laccase engineering: from rational design to directed evolution. Biotechnol Adv 33:25–40
McEwen CN, Layton SF, Taylor SK (1977) Field desorption and electron impact mass spectra of ionic dyes. Anal Chem 49:922–926
Mitrovic J, Radovic M, Bojic D, Andelkovic T, Purenovic M, Bojic A (2012) Decolorization of the textile azo dye reactive orange 16 by the UV/H2O2 process. J Serb Chem Soc 77:465–481
Moosvi S, Keharia H, Madamwar D (2005) Decolourization of textile dye reactive violet 5 by a newly isolated bacterial consortium RVM 11.1. World J Microbiol Biotechnol 21:667–672
Murugesan K, Nam IH, Kim YM, Chang YS (2007) Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enzym Microb Technol 40:1662–1672
Novotny C, Svobodova K, Erbanova P, Cajthaml T, Kasinath A, Lang E, Sasek V (2004) Ligninolytic fungi in bioremediation: extracellular enzyme production and degradation rate. Soil Biol Biochem 36:1545–1551
Papinutti VL, Forchiassin F (2007) Lignocellulolytic enzymes from Fomes sclerodermeus growing in solid-state fermentation. J Food Eng 81:54–59
Park C, Lee M, Lee B, Kim SW, Chase HA, Lee J, Kim S (2007) Biodegradation and biosorption for decolorization of synthetic dyes byFunalia trogii. Biochem EngJ 36:59–65
Pazarbasi MB, Kocyigit A, Ozdemir G, Yasa I, Karaboz I (2012) Decolorization of various leather dyes and leather industry effluent by Trametes trogii TEM H2. Fresenius Environ Bull 21:1410–1416
Przystas W, Godlewska EZ, Sota EG (2012) Biological removal of azo and triphenyl methane dyes and toxicity of process by-products. Water Air Soil Pollut 223:1581–1592
Revankar MS, Lele SS (2006) Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochem 41:581–588
Rosales E, Rodr S, Sanrom MA (2007) Increased laccase production by Trametes hirsuta grown on ground orange peelings. Enzym Microb Technol 40:1286–1290
Salony, Mishra S, Bisaria VS (2006) Production and characterization of laccase from Cyathus bulleri and its use in decolourization of recalcitrant textile dyes. Appl Microbiol Biotechnol 71:646–653
Salvatore MD, Carafa AM, Carratu G (2008) Assessment of heavy metals phytotoxicity using seed germination and root elongation tests: a comparison of two growth substrates. Chemosphere 73:1461–1464
Svobodova K, Senholdt M, Novotny C, Rehorek A (2007) Mechanism of reactive orange 16 degradation with the white rot fungus Irpex lacteus. Process Biochem 42:1279–1284
Telke AA, Kalyani DC, Dawkar VV, Govindwar SP (2009) Influence of organic and inorganic compounds on oxidoreductive decolorization of sulfonated azo dye C.I. reactive orange 16. J Hazard Mater 172:298–309
Vares T, Kalsi M, Hatakka A (1995) Lignin peroxidases, manganese peroxidases and other ligninolytic enzymes produced by Phlebia radiate during solid-state fermentation of wheat straw. Appl Environ Microbiol 61:3515–1320
Wariishi H, Vallis K, Gold MH (1992) Manganese (II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. J Biol Chem 267:23689–23695
Wu SG, Huang L, Head J, Chen DR, Kong I, Tang YJ (2012) Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. J Pet Environ Biotechnol 3:1000126
Acknowledgements
This study was supported by a grant (RP 02875) from Department of Biotechnology, Ministry of Science and Technology (Government of India) awarded to SM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(PDF 221 kb)
Rights and permissions
About this article
Cite this article
Vats, A., Mishra, S. Decolorization of complex dyes and textile effluent by extracellular enzymes of Cyathus bulleri cultivated on agro-residues/domestic wastes and proposed pathway of degradation of Kiton blue A and reactive orange 16. Environ Sci Pollut Res 24, 11650–11662 (2017). https://doi.org/10.1007/s11356-017-8802-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8802-2