Abstract
Urban effluents are rich in nutrients, organic matter, pharmaceuticals and personal care products (PPCPs), pesticides, hydrocarbons, surfactants, and others. Previous studies have shown that oysters Crassostrea gigas accumulate significant levels of linear alkylbenzenes (LABs) in sanitary sewage contaminated sites, but there is little information about its toxicological effects in marine bivalves. The aim of this study was to analyze the transcription of genes in two tissues of C. gigas exposed for 12, 24, and 36 h to LABs or sanitary sewage. Likewise, the activity of antioxidant and biotransformation enzymes was measured in oysters exposed for 36 h in all groups. Oysters exposed to LABs and oysters exposed to sanitary sewage showed different patterns of transcriptional responses. LAB-exposed oysters showed lower level of biological responses than the oysters exposed to sanitary sewage. Despite the ability of the oyster C. gigas to accumulate LABs (28-fold), the data indicate that these contaminants are not the cause for the transcriptional responses observed in oysters exposed to sanitary sewage. Possibly, the biological changes observed in the sanitary sewage-exposed oysters are associated with the presence of other contaminants, which might have caused synergistic, additive, or antagonistic effects. The results show that FABP-like and GST-ω-like messenger RNAs (mRNAs) have a rapid response in tissues of oyster C. gigas exposed to sanitary sewage, suggesting a possible protective response and a role in maintaining homeostasis of these organisms.


Similar content being viewed by others
References
Abessa DMS, Carr RS, Rachid BRF et al (2005) Influence of a Brazilian sewage outfall on the toxicity and contamination of adjacent sediments. Mar Pollut Bull 50:875–885. doi:10.1016/j.marpolbul.2005.02.034
Aebi H (1984) Catalase. In: methods of enzymatic analysis. Academic, London, pp 121–126
Allen JI, Moore MN (2004) Environmental prognostics: is the current use of biomarkers appropriate for environmental risk evaluation? Mar Environ Res 58:227–232. doi:10.1016/j.marenvres.2004.03.119
Ananthan J, Goldberg AL, Voellmy R (1986) Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science 232–522:24
Andrew-Priestley MN, O’Connor WA, Dunstan RH et al (2012) Estrogen mediated effects in the Sydney rock oyster, saccostrea glomerata, following field exposures to sewage effluent containing estrogenic compounds and activity. Aquat Toxicol 120–121:99–108. doi:10.1016/j.aquatox.2012.03.020
Bainy ACD, Saito E, Carvalho PSM, Junqueira VBC (1996) Oxidative stress in gill, erythrocytes, liver and kidney of Nile tilapia (Oreochromis niloticus) from a polluted site. Aquat Toxicol 34:151–162. doi:10.1016/0166-445X(95)00036-4
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological. Environ Toxicol Chem 21:1316–1322
Boonyatumanond R, Wattayakorn G, Togo A, Takada H (2006) Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine, and marine sediments in Thailand. Mar Pollut Bull 52:942–956. doi:10.1016/j.marpolbul.2005.12.015
Boutet I, Tanguy A, Moraga D (2004) Response of the pacific oyster Crassostrea gigas to hydrocarbon contamination under experimental conditions. Gene 329:147–157. doi:10.1016/j.gene.2003.12.027
Brulle F, Cocquerelle C, Mitta G et al (2008) Identification and expression profile of gene transcripts differentially expressed during metallic exposure in Eisenia fetida coelomocytes. Dev Comp Immunol 32:1441–1453. doi:10.1016/j.dci.2008.06.009
Burg D, Riepsaame J, Pont C et al (2006) Peptide-bond modified glutathione conjugate analogs modulate GSTpi function in GSH-conjugation, drug sensitivity and JNK signaling. Biochem Pharmacol 71:268–277. doi:10.1016/j.bcp.2005.11.003
Burns KA, Smith JL (1981) Biological monitoring of ambient water quality: the case for using bivalves as sentinel organisms for monitoring petroleum pollution in coastal waters. Estuar Coast Shelf Sci 13:433–443. doi:10.1016/S0302-3524(81)80039-4
Cajaraville MP, Bebianno MJ, Blasco J et al (2000) The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: a practical approach. Sci Total Environ 247:295–311
Capuzzo JM (1996) The bioaccumulation and biological effects of lipophilic organic contaminants. In: Kennedy VS, Newell RE, Eble AF (eds) The eastern oyster: Crassostrea virginica. Maryland Sea Grant College, Silverspring, pp 539–557
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Cheng P, Liu X, Zhang G, He J (2007) Cloning and expression analysis of a HSP70 gene from Pacific abalone (Haliotis discus hannai). Fish Shellfish Immunol 22:77–87. doi:10.1016/j.fsi.2006.03.014
Cheung CC, Zheng GJ, Li AM et al (2001) Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquat Toxicol 52:189–203
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–762. doi:10.1016/j.freeradbiomed.2009.12.022
Comoglio L, Amin O, Botté S, Marcovecchio J (2011) Use of biomarkers in resident organisms as a tool for environmental monitoring in a cold coastal system, Tierra del Fuego Island. Ecotoxicol Environ Saf 74:382–393. doi:10.1016/j.ecoenv.2010.10.005
Corcoran E, Nellemann C, Baker E et al (2010) Sick water? The central role of wastewater management in sustainable development - a rapid response assessment. Birkeland Trykkeri, Norway
Cossu C, Doyotte A, Babut M et al (2000) Antioxidant biomarkers in freshwater bivalves, Unio tumidus, in response to different contamination profiles of aquatic sediments. Ecotoxicol Environ Saf 45:106–121. doi:10.1006/eesa.1999.1842
Edser C (2012) Feedstock sourcing looks to the emerging markets. Focus surfactants 1–2
Eganhouse RP, Blumfield DL, Kaplan IR (1983) Long-chain alkylbenzenes as molecular tracers of domestic wastes in the marine environment. Environ Sci Technol 17:523–530. doi:10.1021/es00115a006
Esteves A, Ehrlich R (2006) Invertebrate intracellular fatty acid binding proteins. Comp Biochem Physiol C Toxicol Pharmacol 142:262–274. doi:10.1016/j.cbpc.2005.11.006
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282. doi:10.1146/annurev.physiol.61.1.243
Fowler BA (2005) Molecular biomarkers: challenges and prospects for the future. Toxicol Appl Pharmacol 206:97. doi:10.1016/j.taap.2005.05.013
Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7:489–503. doi:10.1038/nrd2589
Gagné F, Blaise C, Pellerin J, André C (2007) Neuroendocrine disruption in Mya arenaria clams during gametogenesis at sites under pollution stress. Mar Environ Res 64:87–107. doi:10.1016/j.marenvres.2006.12.014
Glock GE, McLean P (1953) Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J 55:400–408
Gunther AJ, Davis JAYA, Hardin DD et al (1999) Long-term bioaccumulation monitoring with transplanted bivalves in the San Francisco estuary. Mar Pollut Bull 38:170–181
Halliwell B, Gutteridge JM (2007) Free radicals in biology and medicine. Oxford University Press, New York
Ho H, Cheng M, Shiao M, Chiu DT (2013) Free radical biology and medicine characterization of global metabolic responses of glucose-6-phosphate dehydrogenase-deficient hepatoma cells to diamide-induced oxidative stress. Free Radic Biol Med 54:71–84
Hoarau P, Damiens G, Roméo M et al (2006) Cloning and expression of a GST-pi gene in Mytilus galloprovincialis. Attempt to use the GST-pi transcript as a biomarker of pollution. Comp Biochem Physiol C Toxicol Pharmacol 143:196–203. doi:10.1016/j.cbpc.2006.02.007
Hunter CL, Stephenson MD, Tjeerdema RS et al (1995) Contaminants in oysters in Kaneohe Bay, Hawaii. Mar Pollut Bull 30:646–654
Isobe KO, Zakaria MP, Chiem NH et al (2004) Distribution of linear alkylbenzenes (LABs) in riverine and coastal environments in South and Southeast Asia. Water Res 38:2448–2458. doi:10.1016/j.watres.2004.02.009
Ju Z, Wells MC, Heater SJ, Walter RB (2007) Multiple tissue gene expression analyses in Japanese medaka (Oryzias latipes) exposed to hypoxia. Comp Biochem Physiol C Toxicol Pharmacol 145:134–144. doi:10.1016/j.cbpc.2006.06.012
Keen JH, Habig WH, Jakoby WB (1976) Mechanism for the several activities of the glutathione S-transferase. J Biol Chem 251:6183–6188
Kim M, Ahn I-Y, Cheon J, Park H (2009) Molecular cloning and thermal stress-induced expression of a pi-class glutathione S-transferase (GST) in the antarctic bivalve Laternula elliptica. Comp Biochem Physiol A Mol Integr Physiol 152:207–213. doi:10.1016/j.cbpa.2008.09.028
Kletzien RF, Harris PKW, Foellmi LA (1994) Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J 8:174–181
Konings AW, Penninga P (1985) On the importance of the level of glutathione and the activity of the pentose phosphate pathway in heat sensitivity and thermotolerance. Int J Radiat Biol Relat Stud Physics Chem Med 48:409–422
Lüchmann KH, Mattos JJ, Siebert MN et al (2011) Biochemical biomarkers and hydrocarbons concentrations in the mangrove oyster Crassostrea brasiliana following exposure to diesel fuel water-accommodated fraction. Aquat Toxicol 105:652–660. doi:10.1016/j.aquatox.2011.09.003
Macías-Zamora JV, Ramírez-Alvarez N (2004) Tracing sewage pollution using linear alkylbenzenes (LABs) in surface sediments at the south end of the Southern California Bight. Environ Pollut 130:229–238. doi:10.1016/j.envpol.2003.12.004
Macleod WD et al. (1986) Extractable toxic organic components In: standard analytical procedures of the NOAA national analytical facility, 1985–1986. 2rd edn. U.S. Department of Commerce, NOAA/NMFS Tech. Memo. NMFS F/NWC-92, p 121
Mao Y, Zhou Y, Yang H, Wang R (2006) Seasonal variation in metabolism of cultured Pacific oyster, Crassostrea gigas, in Sanggou Bay, China. Aquaculture 253:322–333. doi:10.1016/j.aquaculture.2005.05.033
Marigómez I, Zorita I, Izagirre U et al (2013) Combined use of native and caged mussels to assess biological effects of pollution through the integrative biomarker approach. Aquat Toxicol 136–137:32–48. doi:10.1016/j.aquatox.2013.03.008
Mccord JM, Fridovich I (1969) Superoxide dismutase: an enzymatic function for erythrocuprein (Hemocuprein). J Biol Chem 244:6049–6055
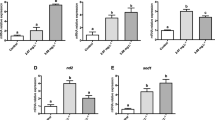
Medeiros ID, Siebert MN, Toledo-Silva G et al (2008a) Differential gene expression in oyster exposed to sewage. Mar Environ Res 66:156–157. doi:10.1016/j.marenvres.2008.02.048
Medeiros ID, Siebert MN, Toledo-Silva G et al (2008b) Induced gene expression in oyster Crassostrea gigas exposed to sewage. Environ Toxicol Pharmacol 26:362–365. doi:10.1016/j.etap.2008.05.004
Miao J, Pan L, Liu N et al (2011) Molecular cloning of CYP4 and GSTpi homologues in the scallop Chlamys farreri and its expression in response to benzo[a]pyrene exposure. Mar Genomics 4:99–108. doi:10.1016/j.margen.2011.03.002
Moore MN, Depledge MH, Readman JW, Paul Leonard DR (2004) An integrated biomarker-based strategy for ecotoxicological evaluation of risk in environmental management. Mutat Res 552:247–268. doi:10.1016/j.mrfmmm.2004.06.028
Mukhopadhyay I et al (2003) Heat shock response: hsp70 in environmental monitoring. J Biochem Mol Toxicol 17:246–254
Oehlmann J, Schulte-Oehlmann U (2003) Chapter 17—molluscs as bioindicators. Trace Metals Other Contam Environ Bioindic Biomonitors Princ Concepts Appl 6:577–635
Peterson GL (1977) A simplification of the protein assay method of lowry et al. Which is more generally applicable. Anal Biochem 356:346–356
Raymundo CC, Preston MR (1992) The distribution of linear alkylbenzenes in coastal and estuarine sediments of the Western North sea. Mar Pollut Bull 24:138–146
Regoli F, Pellegrini D, Winston GW et al (2002) Application of biomarkers for assessing the biological impact of dredged materials in the Mediterranean: the relationship between antioxidant responses and susceptibility to oxidative stress in the red mullet (Mullus barbatus). Mar Pollut Bull 44:912–922
Rewitz KF, Styrishave B, Løbner-Olsen A, Andersen O (2006) Marine invertebrate cytochrome P450: emerging insights from vertebrate and insects analogies. Comp Biochem Physiol C Toxicol Pharmacol 143:363–381. doi:10.1016/j.cbpc.2006.04.001
Richardson BJ, Mak E, De Luca-Abbott SB et al (2008) Antioxidant responses to polycyclic aromatic hydrocarbons and organochlorine pesticides in green-lipped mussels (Perna viridis): do mussels “integrate” biomarker responses? Mar Pollut Bull 57:503–514. doi:10.1016/j.marpolbul.2008.02.032
Rinawati KT, Koike H et al (2012) Distribution, source identification, and historical trends of organic micropollutants in coastal sediment in Jakarta Bay, Indonesia. J Hazard Mater 217–218:208–216. doi:10.1016/j.jhazmat.2012.03.023
Robinson EC, Nair RS (1992) The genotoxic potential of linear alkylbenzene mixtures in a short-term test battery. Fundam Appl Toxicol 18:540–548
Robinson EC, Schroeder RE (1992) Reproductive and developmental toxicity studies of a linear alkylbenzene mixture in rats. Fundam Appl Toxicol 18:549–556
Rodríguez-Ortega MJ et al (2002) Biochemical biomarkers os pollution on the clam Chamaelea gallina from south-spanish littoral. Environ Toxicol Chem 21:542–549
Ryan JA, Hightower LE (1996) Stress proteins as molecular biomarkers for environmental toxicology. Stress Cell Responses –. EXS 77:411–424
Saavedra C, Bachère E (2006) Bivalve genomics. Aquaculture 256:1–14. doi:10.1016/j.aquaculture.2006.02.023
Sáenz LA, Seibert EL, Zanette J et al (2010) Biochemical biomarkers and metals in Perna perna mussels from mariculture zones of Santa Catarina, Brazil. Ecotoxicol Environ Saf 73:796–804. doi:10.1016/j.ecoenv.2010.02.015
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. doi:10.1038/nprot.2008.73
Serafim CFS, Hazin F (2006) The coastal ecosystem. In: Chaves PT (ed) Geography: the sea in geographic space. Ministry of Education, Department of Education, Brasília p, p 103 (In portuguese)
Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem Biophys Res Commun 360:1–16
Sherblom PM, Gschwend PM, Eganhouse RP (1992) Aqueous solubilities, vapor pressures, and 1-octanol-water partition coefficients for C9-C14 linear. J Chem Eng Data 37:394–339
Sies H (1991) Oxidative stress: from basic research to clinical application. Am J Med 91:31S–38S
Sies H, Stahl W, Sundquist AR (1992) Antioxidant functions of vitamins—vitamin-E and vitamin-C, beta-carotene, and other carotenoids. Ann N Y Acad Sci 669:7–20
Siritantikorn A, Johansson K, Ahlen K et al (2007) Protection of cells from oxidative stress by microsomal glutathione transferase 1. Biochem Biophys Res Commun 355:592–596. doi:10.1016/j.bbrc.2007.02.018
Song L, Wu L, Ni D et al (2006) The cDNA cloning and mRNA expression of heat shock protein 70 gene in the haemocytes of bay scallop (Argopecten irradians, Lamarck 1819) responding to bacteria challenge and naphthalin stress. Fish Shellfish Immunol 21:335–345. doi:10.1016/j.fsi.2005.12.011
Souza DSM, Ramos APD, Flores-Nunes F et al (2012) Evaluation of tropical water sources and mollusks in southern Brazil using microbiological, biochemical, and chemical parameters. Ecotoxicol Environ Saf 76:153–161. doi:10.1016/j.ecoenv.2011.09.018
Storch J, Thumser AE (2000) The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta 1486:28–44
Storey KB (1996) Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 29:1715–1733
Takada H, Ishiwatari R, Ogura N (1992) Distribution of linear alkylbenzenes (LABs) and linear alkylbenzene sulphonates (LAS) in Tokyo Bay sediments. Estuar Coast Shelf Sci 35:141–156
Toledo-Silva G, Siebert MN, Medeiros ID et al (2008) Cloning a new cytochrome P450 isoform (CYP356A1) from oyster Crassostrea gigas. Mar Environ Res 66:15–18. doi:10.1016/j.marenvres.2008.02.010
Travers M-A, Meistertzheim A-L, Cardinaud M et al (2010) Gene expression patterns of abalone, Haliotis tuberculata, during successive infections by the pathogen Vibrio harveyi. J Invertebr Pathol 105:289–297. doi:10.1016/j.jip.2010.08.001
Tsutsumi S, Yamaguchi Y, Nishida I et al (2002) Alkylbenzenes in mussels from South and South East Asian coasts as a molecular tool to assess sewage impact. Mar Pollut Bull 45:325–331
Ursini MV, Parrella A, Rosa G et al (1997) Enhanced expression of glucose-6-phosphate dehydrogenase in human cells sustaining oxidative stress. Biochem J 323:801–806
Venkatesan MI, Northrup T, Phillip CR (2002) Determination of linear alkylbenzenes in fish tissue by gel permeation chromatography and gas chromatography–mass spectrometry. J Chromatogr A 942:223–230
Verlecar XN, Jena KB, Chainy GBN (2008) Modulation of antioxidant defences in digestive gland of Perna viridis (L.), on mercury exposures. Chemosphere 71:1977–1985. doi:10.1016/j.chemosphere.2007.12.014
Voisine C, Orton K, Morimoto RI (2007) Protein misfolding, chaperone networks, and the heat shock response in the nervous system. In: Waxman SG (Ed.). Molecular neurology. [s.l.] Elsevier Academic Press, pp 60–76
Walker CH , Sibly RM, Hopkin SP et al (2001) Principles of ecotoxicology. Taylor & Francis, Londres
Wang Z, Wu Z, Jian J, Lu Y (2009) Cloning and expression of heat shock protein 70 gene in the haemocytes of pearl oyster (Pinctada fucata, Gould 1850) responding to bacterial challenge. Fish Shellfish Immunol 26:639–645. doi:10.1016/j.fsi.2008.10.011
Wang K, Deng G, Chen G et al (2012) Heat shock protein 70 inhibits hydrogen peroxide-induced nucleolar fragmentation via suppressing cleavage and down-regulation of nucleolin. Cell Stress Chaperones 17:121–130. doi:10.1007/s12192-011-0292-4
Wendel A (1981) Glutathione peroxidase. Method Enzymol 77:325–333
Xu C, Pan L, Liu N et al (2010) Cloning, characterization and tissue distribution of a pi-class glutathione S-transferase from clam (Venerupis philippinarum): response to benzo[alpha]pyrene exposure. Comp Biochem Physiol C Toxicol Pharmacol 152:160–166. doi:10.1016/j.cbpc.2010.03.011
Yue X, Liu B, Sun L, Tang B (2011) Cloning and characterization of a hsp70 gene from Asiatic hard clam Meretrix meretrix which is involved in the immune response against bacterial infection. Fish Shellfish Immunol 30:791–799. doi:10.1016/j.fsi.2010.12.027
Zanette J, Flores-Nunes F, Medeiros ID et al (2008) Comparison of the antioxidant defense system in Crassostrea rhizophorae and Crassostrea gigas exposed to domestic sewage discharges. Mar Environ Res 66:196–198. doi:10.1016/j.marenvres.2008.02.057
Zanette J, Goldstone JV, Bainy ACD, Stegeman JJ (2010) Identification of CYP genes in Mytilus (mussel) and Crassostrea (oyster) species: first approach to the full complement of cytochrome P450 genes in bivalves. Mar Environ Res 69:S1–S3. doi:10.1016/j.marenvres.2009.10.013.Identification
Zhang K, Wang J-Z, Liang B et al (2012a) Assessment of aquatic wastewater pollution in a highly industrialized zone with sediment linear alkylbenzenes. Environ Toxicol Chem 31:724–730. doi:10.1002/etc.1768
Zhang G, Fang X, Guo X et al (2012b) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490:49–54. doi:10.1038/nature11413
Acknowledgments
During this work, FFN received a PhD fellowship CNPq (# 145331/2009-1). The research leading to these results has received funding from the EU project GENERA within the framework of the Marie Curie IRSES Actions (FP7-PEOPLE-2009-IRSES—Proposal No. 247559), CNPq (#483028/2012-6), and National Institute of Science and Technology-Aquatic Toxicology (INCT-TA). Analysis of organic contaminants was conducted in partnership with the Laboratory of Organic Chemistry of the Oceanographic Institute of the University of São Paulo (LQO-IO-USP). ACDB, MCB, and CMRM are recipients of CNPq productivity fellowships.
Compliance with ethical standards
ᅟ
Conflict of interest
The authors declare the inexistence of any conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Linear alkylbenzene (LAB) isomers accumulated in tissues of oyster Crassostrea gigas after 36 h of exposure to different experimental conditions (ng.g-1). (DOCX 18 kb)
Supplementary Fig. 2
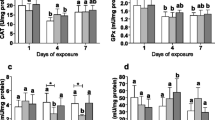
Biochemical responses of the enzymes analyzed in gill and digestive gland of Crassostrea gigas after 36 h of exposure to treatments. CT: clean sea water control; SEW: sanitary sewage 33 %; SOL: solvent control; LAB: nominal concentration of 40 μg.L-1 mixtures of linear alkylbenzenes (p < 0.05). CAT: catalase; GPx: glutathione peroxidase. (PDF 37 kb)
Rights and permissions
About this article
Cite this article
Flores-Nunes, F., Mattos, J.J., Zacchi, F.L. et al. Effect of linear alkylbenzene mixtures and sanitary sewage in biochemical and molecular responses in pacific oyster Crassostrea gigas . Environ Sci Pollut Res 22, 17386–17396 (2015). https://doi.org/10.1007/s11356-015-4486-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4486-7