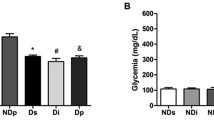
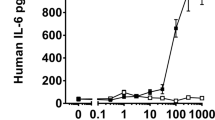
Abstract
In addition to their role in glycosylation reactions, UDP-sugars are released from cells and activate widely distributed cell surface P2Y14 receptors (P2Y14R). However, the physiological/pathophysiological consequences of UDP-sugar release are incompletely defined. Here, we report that UDP-glucose levels are abnormally elevated in lung secretions from patients with cystic fibrosis (CF) as well as in a mouse model of CF-like disease, the βENaC transgenic (Tg) mouse. Instillation of UDP-glucose into wild-type mouse tracheas resulted in enhanced neutrophil lung recruitment, and this effect was nearly abolished when UDP-glucose was co-instilled with the P2Y14R antagonist PPTN [4-(piperidin-4-yl)-phenyl)-7-(4-(trifluoromethyl)-phenyl-2-naphthoic acid]. Importantly, administration of PPTN to βENaC-Tg mice reduced neutrophil lung inflammation. These results suggest that UDP-glucose released into the airways acts as a local mediator of neutrophil inflammation.





Similar content being viewed by others
References
Burnstock G (2006) Purinergic signalling. Br J Pharmacol 147(Suppl 1):S172–S181
Lazarowski ER (2012) Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal 8:359–373
Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341
Khakh BS, North RA (2006) P2X receptors as cell-surface ATP sensors in health and disease. Nature 442:527–532
Lazarowski ER, Boucher RC (2009) Purinergic receptors in airway epithelia. Curr Opin Pharmacol 9:262–267
Boucher RC (2003) Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch 445:495–498
Idzko M, Ferrari D, Eltzschig HK (2014) Nucleotide signalling during inflammation. Nature 509:310–317
Ribeiro CM, Paradiso AM, Carew MA, Shears SB, Boucher RC (2005) Cystic fibrosis airway epithelial Ca2+ i signaling: the mechanism for the larger agonist-mediated Ca2+ i signals in human cystic fibrosis airway epithelia. J Biol Chem 280:10202–10209
Douillet CD, Robinson WP III, Milano PM, Boucher RC, Rich PB (2006) Nucleotides induce IL-6 release from human airway epithelia via P2Y2 and p38 MAPK-dependent pathways. Am J Physiol Lung Cell Mol Physiol 291:L734–L746
Muller T, Bayer H, Myrtek D, Ferrari D, Sorichter S, Ziegenhagen MW, Zissel G, Virchow Jr JC, Luttmann W, Norgauer J, Di Virgilio F, Idzko M (2005) The P2Y14 receptor of airway epithelial cells: coupling to intracellular Ca2+ and IL-8 secretion. Am J Respir Cell Mol Biol 33:601–609
Downey DG, Bell SC, Elborn JS (2009) Neutrophils in cystic fibrosis. Thorax 64:81–88
Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, Trill J, Swift A, Aiyar N, Taylor P, Vawter L, Naheed S, Szekeres P, Hervieu G, Scott C, Watson JM, Murphy AJ, Duzic E, Klein C, Bergsma DJ, Wilson S, Livi GP (2000) A G protein-coupled receptor for UDP-glucose. J Biol Chem 275:10767–10771
Lazarowski ER, Harden TK (2015) UDP-sugars as extracellular signaling molecules: cellular and physiologic consequences of P2Y14 receptor activation. Mol Pharmacol 88:151–160
Carter RL, Fricks IP, Barrett MO, Burianek LE, Zhou Y, Ko H, Das A, Jacobson KA, Lazarowski ER, Harden TK (2009) Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Mol Pharmacol 76:1341–1348
Harden TK, Sesma JI, Fricks IP, Lazarowski ER (2010) Signalling and pharmacological properties of the P2Y14 receptor. Acta Physiol (Oxford) 199:149–160
Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, Szekeres PG, Wilson S, Freeman KB, Emson PC (2003) GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res 118:10–23
Skelton L, Cooper M, Murphy M, Platt A (2003) Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol 171:1941–1949
Lee BC, Cheng T, Adams GB, Attar EC, Miura N, Lee SB, Saito Y, Olszak I, Dombkowski D, Olson DP, Hancock J, Choi PS, Haber DA, Luster AD, Scadden DT (2003) P2Y-like receptor, GPR105 (P2Y14), identifies and mediates chemotaxis of bone-marrow hematopoietic stem cells. Genes Dev 17:1592–1604
Gao ZG, Ding Y, Jacobson KA (2010) UDP-glucose acting at P2Y14 receptors is a mediator of mast cell degranulation. Biochem Pharmacol 79:873–879
Scrivens M, Dickenson JM (2006) Functional expression of the P2Y(14) receptor in human neutrophils. Eur J Pharmacol 543:166–173
Okada SF, Zhang L, Kreda SM, Abdullah LH, Davis CW, Pickles RJ, Lazarowski ER, Boucher RC (2011) Coupled nucleotide and mucin hypersecretion from goblet cell metaplastic human airway epithelium. Am J Respir Cell Mol Biol 45:253–260
Lazarowski ER (2010) Quantification of extracellular UDP-galactose. Anal Biochem 396:23–29
Sesma JI, Esther CR Jr, Kreda SM, Jones L, O’Neal W, Nishihara S, Nicholas RA, Lazarowski ER (2009) ER/golgi nucleotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J Biol Chem 284:12572–12583
Kreda SM, Seminario-Vidal L, Heusden C, Lazarowski ER (2008) Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol 153:1528–1537
Esther CR Jr, Sesma JI, Dohlman HG, Ault AD, Clas ML, Lazarowski ER, Boucher RC (2008) Similarities between UDP-glucose and adenine nucleotide release in yeast: involvement of the secretory pathway. Biochemistry 47:9269–9278
Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER (2007) Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol 584:245–259
Lazarowski ER, Shea DA, Boucher RC, Harden TK (2003) Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol 63:1190–1197
Sesma JI, Kreda SM, Steinckwich-Besancon N, Dang H, Garcia-Mata R, Harden TK, Lazarowski ER (2012) The UDP-sugar-sensing P2Y14 receptor promotes Rho-mediated signaling and chemotaxis in human neutrophils. Am J Phys Cell Physiol 303:C490–C498
Barrett MO, Sesma JI, Ball CB, Jayasekara PS, Jacobson KA, Lazarowski ER, Harden TK (2013) A selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils. Mol Pharmacol 84:41–49
Loughlin CE, Esther CR Jr, Lazarowski ER, Alexis NE, Peden DB (2010) Neutrophilic inflammation is associated with altered airway hydration in stable asthmatics. Respir Med 104:29–33
Esther CR, Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Pedrosa Ribeiro CM, Moore CG, Davis SD, Boucher RC (2008) Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur Respir J 31:949–956
Lazarowski ER, Boucher RC, Harden TK (2000) Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem 275:31061–31068
Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC (2004) Increased airway epithelial Na + absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10:487–493
Jones LC, Moussa L, Fulcher ML, Zhu Y, Hudson EJ, O’Neal WK, Randell SH, Lazarowski ER, Boucher RC, Kreda SM (2012) VAMP8 is a vesicle SNARE that regulates mucin secretion in airway goblet cells. J Physiol 590:545–562
Livraghi A, Grubb BR, Hudson EJ, Wilkinson KJ, Sheehan JK, Mall MA, O’Neal WK, Boucher RC, Randell SH (2009) Airway and lung pathology due to mucosal surface dehydration in {beta}-epithelial Na + channel-overexpressing mice: role of TNF-{alpha} and IL-4R{alpha} signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J Immunol 182:4357–4367
Zhou Z, Treis D, Schubert SC, Harm M, Schatterny J, Hirtz S, Duerr J, Boucher RC, Mall MA (2008) Preventive but not late amiloride therapy reduces morbidity and mortality of lung disease in betaENaC-overexpressing mice. Am J Respir Crit Care Med 178:1245–1256
Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S, Fuller F, Almond M, Qaqish B, Bordonali E, Rubinstein M, Bennett WD, Kesimer M, Boucher RC (2015) The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med 192:180–190
Picher M, Burch LH, Boucher RC (2004) Metabolism of P2 receptor agonists in human airways: implications for mucociliary clearance and cystic fibrosis. J Biol Chem 279:20234–20241
Fausther M, Pelletier J, Ribeiro CM, Sevigny J, Picher M (2010) Cystic fibrosis remodels the regulation of purinergic signaling by NTPDase1 (CD39) and NTPDase3. Am J Physiol Lung Cell Mol Physiol 298:L804–L818
Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, O’Neal WK, Boucher RC (2008) Development of chronic bronchitis and emphysema in beta-epithelial Na + channel-overexpressing mice. Am J RespirCrit Care Med 177:730–742
Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, Randell SH, O’Neal WK (2012) Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol 5:397–408
Evans CM, Koo JS (2009) Airway mucus: the good, the bad, the sticky. Pharmacol Ther 121:332–348
Yoshida T, Tuder RM (2007) Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 87:1047–1082
Rollins BM, Burn M, Coakley RD, Chambers LA, Hirsh AJ, Clunes MT, Lethem MI, Donaldson SH, Tarran R (2008) A2B adenosine receptors regulate the mucus clearance component of the lung’s innate defense system. Am J Respir Cell Mol Biol 39:190–197
Freeman K, Tsui P, Moore D, Emson PC, Vawter L, Naheed S, Lane P, Bawagan H, Herrity N, Murphy K, Sarau HM, Ames RS, Wilson S, Livi GP, Chambers JK (2001) Cloning, pharmacology, and tissue distribution of G-protein-coupled receptor GPR105 (KIAA0001) rodent orthologs. Genomics 78:124–128
Arase T, Uchida H, Kajitani T, Ono M, Tamaki K, Oda H, Nishikawa S, Kagami M, Nagashima T, Masuda H, Asada H, Yoshimura Y, Maruyama T (2009) The UDP-glucose receptor P2RY14 triggers innate mucosal immunity in the female reproductive tract by inducing IL-8. J Immunol 182:7074–7084
Saini Y, Dang H, Livraghi-Butrico A, Kelly EJ, Jones LC, O’Neal WK, Boucher RC (2014) Gene expression in whole lung and pulmonary macrophages reflects the dynamic pathology associated with airway surface dehydration. BMC Genomics 15:726
Zimmermann H, Zebisch M, Strater N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502
Zuo P, Picher M, Okada SF, Lazarowski ER, Button B, Boucher RC, Elston TC (2008) Mathematical model of nucleotide regulation on airway epithelia. Implications for airway homeostasis. J Biol Chem 283:26805–26819
Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG (2008) Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem 283:28480–28486
Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC (2003) Ecto 5′-nucleotidase and non-specific alkaline phosphatase: two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem 278:13468–13479
Seminario-Vidal L, van Heusden C, Mugesh G, Lazarowski ER (2010) Ebselen is a potent non-competitive inhibitor of extracellular nucleoside diphosphokinase. Purinergic Signal 6:383–391
Fricks IP, Carter RL, Lazarowski ER, Harden TK (2009) Gi-dependent cell signaling responses of the human P2Y14-receptor in model cell systems. J Pharmacol Exp Ther 330:162–168
Acknowledgments
The authors are grateful to Dr. Silvia Kreda and Dr. Michael Chua (Histology/Microscopy Core) for the use of the Nikon Nicrophot-SA microscopy, Dr. Wanda O’Neal and Kristen Wilkinson (Mouse Core) for assisting with the βENaC-Tg mouse studies, and to Catharina van Heusden for technical assistance with the use of the HPLC.
This work was supported by the National Institutes of Health (NIH) National Institute of General Medical Sciences [R01-GM38213] (TKH), the NIH National Heart, Lung, and Blood Institute [P01-HL110873] (ERL), the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program (KAJ), the NIH Specialized Centers of Clinically Orientated Research [P50-HL084934] (SD), the US EPA cooperative agreement CR 833463015-35475 and NHLBI-RO1 HL080337 (NEA), and the American Cystic Fibrosis Foundation [SESMA1510] and North Carolina Translational & Clinical Science [NCTraCS 550KR101513] (JIS). The Histology/Microscopy and Mouse Cores were supported by NIH P30-DK065988.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All subjects were studied at the University of North Carolina at Chapel Hill (Chapel Hill, NC, USA), and studies were approved by the Institutional Review Board. Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Supplemental Figure 1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Sesma, J.I., Weitzer, C.D., Livraghi-Butrico, A. et al. UDP-glucose promotes neutrophil recruitment in the lung. Purinergic Signalling 12, 627–635 (2016). https://doi.org/10.1007/s11302-016-9524-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-016-9524-5