Abstract
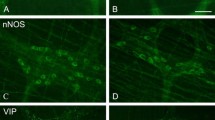
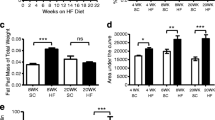
Gastrointestinal symptoms have a major impact on the quality of life and are becoming more prevalent in the western population. The enteric nervous system (ENS) is pivotal in regulating gastrointestinal functions. Purinergic neurotransmission conveys a range of short and long-term cellular effects. This study investigated the role of the ADP-sensitive P2Y13 receptor in lipid-induced enteric neuropathy. Littermate P2Y13 +/+ and P2Y13 −/− mice were fed with either a normal diet (ND) or high-fat diet (HFD) for 6 months. The intestines were analysed for morphological changes as well as neuronal numbers and relative numbers of vasoactive intestinal peptide (VIP)- and neuronal nitric oxide synthase (nNOS)-containing neurons. Primary cultures of myenteric neurons from the small intestine of P2Y13 +/+ or P2Y13 −/− mice were exposed to palmitic acid (PA), the P2Y13 receptor agonist 2meSADP and the antagonist MRS2211. Neuronal survival and relative number of VIP-containing neurons were analysed. In P2Y13 +/+, but not in P2Y13 −/− mice, HFD caused a significant loss of myenteric neurons in both ileum and colon. In colon, the relative numbers of VIP-containing submucous neurons were significantly lower in the P2Y13 −/− mice compared with P2Y13 +/+ mice. The relative numbers of nNOS-containing submucous colonic neurons increased in P2Y13 +/+ HFD mice. HFD also caused ileal mucosal thinning in P2Y13 +/+ and P2Y13 −/− mice, compared to ND fed mice. In vitro PA exposure caused loss of myenteric neurons from P2Y13 +/+ mice while neurons from P2Y13 −/− mice were unaffected. Presence of MRS2211 prevented PA-induced neuronal loss in cultures from P2Y13 +/+ mice. 2meSADP caused no change in survival of cultured neurons. P2Y13 receptor activation is of crucial importance in mediating the HFD- and PA-induced myenteric neuronal loss in mice. In addition, the results indicate a constitutive activation of enteric neuronal apoptosis by way of P2Y13 receptor stimulation.



Similar content being viewed by others
References
Frank L, Kleinman L, Ganoczy D et al (2000) Upper gastrointestinal symptoms in North America—prevalence and relationship to healthcare utilization and quality of life. Dig Dis Sci 45:809–818
Tougas G, Chen Y, Hwang P, Liu MM, Eggleston A (1999) Prevalence and impact of upper gastrointestinal symptoms in the Canadian population: findings from the DIGEST study. Am J Gastroenterol 94:2845–2854
Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M (2001) Prevalence of gastrointestinal symptoms associated with diabetes mellitus—a population-based survey of 15,000 adults. Arch Intern Med 161:1989–1996
Burnstock G, Campbell G, Satchell D, Smythe A (1970) Evidence that adenosine triphosphate or a related nucleotide is transmitter substance released by non-adrenergic inhibitory nerves in gut. Br J Pharmacol 40:668
Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H (2009) Purinergic signalling in the nervous system: an overview. Trends Neurosci 32:19–29
Burnstock G, Cocks T, Kasakov L, Wong HK (1978) Direct evidence for ATP release from non-adrenergic, non-cholinergic (purinergic) nerves in guinea-pig taenia-coli and bladder. Eur J Pharmacol 49:145–149
Abbracchio MP, Burnstock G (1998) Purinergic signalling: pathophysiological roles. Jpn J Pharmacol 78:113–145
Zimmermann H (1996) Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol 49:589–618
Gourine AV, Wood JD, Burnstock G (2009) Purinergic signalling in autonomic control. Trends Neurosci 32:241–248
Communi D, Gonzalez NS, Detheux M et al (2001) Identification of a novel human ADP receptor coupled to G(i). J Biol Chem 276:41479–41485
Malin SA, Molliver DC (2010) Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain 6:12
Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K (2012) Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia 60:1529–1539
Jacquet S, Malaval C, Martinez LO et al (2005) The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell Mol Life Sci 62:2508–2515
Gao ZG, Ding Y, Jacobson KA (2010) P2Y(13) receptor is responsible for ADP-mediated degranulation in RBL-2H3 rat mast cells. Pharmacol Res 62:500–505
Amisten S, Meidute-Abaraviciene S, Tan C et al (2010) ADP mediates inhibition of insulin secretion by activation of P2Y13 receptors in mice. Diabetologia 53:1927–1934
Yano S, Tsukimoto M, Harada H, Kojima S (2012) Involvement of P2Y13 receptor in suppression of neuronal differentiation. Neurosci Lett 518:5–9
del Puerto A, Diaz-Hernandez JI, Tapia M et al (2012) Adenylate cyclase 5 coordinates the action of ADP, P2Y1, P2Y13 and ATP-gated P2X7 receptors on axonal elongation. J Cell Sci 125:176–188
Espada S, Ortega F, Molina-Jijon E et al (2010) The purinergic P2Y(13) receptor activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Free Radic Biol Med 49:416–426
Fabre AC, Malaval C, Ben Addi A et al (2010) P2Y13 receptor is critical for reverse cholesterol transport. Hepatology 52:1477–1483
Blom D, Yamin TT, Champy MF et al (2010) Altered lipoprotein metabolism in P2Y(13) knockout mice. Biochim Biophys Acta 1801:1349–1360
Serhan N, Cabou C, Verdier C et al (2013) Chronic pharmacological activation of P2Y(13) receptor in mice decreases HDL-cholesterol level by increasing hepatic HDL uptake and bile acid secretion. Biochim Biophys Acta 1831:719–725
Voss U, Sand E, Olde B, Ekblad E (2013) Enteric neuropathy can be induced by high fat diet in vivo and palmitic acid exposure in vitro. PloS One 8(12):e81413
Brommage R (2003) Validation and calibration of DEXA body composition in mice. Am J Physiol Endocrinol Metab 285:E454–E459
Sand E, Themner-Persson A, Ekblad E (2009) Mast cells reduce survival of myenteric neurons in culture. Neuropharmacology 56:522–530
Voss U, Sand E, Hellström PM, Ekblad E (2012) Glucagon-like peptides 1 and 2 and vasoactive intestinal peptide are neuroprotective on cultured and mast cell co-cultured rat myenteric neurons. BMC Gastroenterol 12:30
Kvietys PR, Specian RD, Grisham MB, Tso P (1991) Jejunal mucosal injury and restitution—role of hydrolytic products of food digestion. Am J Physiol 261:G384–G391
Falktoft B, Georg B, Fahrenkrug J (2009) Signaling pathways in PACAP regulation of VIP gene expression in human neuroblastoma cells. Neuropeptides 43:387–396
Ekblad E, Mulder H, Sundler F (1996) Vasoactive intestinal peptide expression in enteric neurons is upregulated by both colchicine and axotomy. Regul Pept 63:113–121
Lin Z, Sandgren K, Ekblad E (2003) Increased expression of vasoactive intestinal polypeptide in cultured myenteric neurons from adult rat small intestine. Auton Neurosci 107:9–19
Sandgren K, Lin Z, Svenningsen AF, Ekblad E (2003) Vasoactive intestinal peptide and nitric oxide promote survival of adult rat myenteric neurons in culture. J Neurosci Res 72:595–602
Lin Z, Sandgren K, Ekblad E (2004) Increased expression of nitric oxide synthase in cultured neurons from adult rat colonic submucous ganglia. Auton Neurosci 114:29–38
Young HM, Ciampoli D (1998) Transient expression of neuronal nitric oxide synthase by neurons of the submucous plexus of the mouse small intestine. Cell Tissue Res 291:395–401
Antonioli L, Colucci R, Pellegrini C et al (2013) The role of purinergic pathways in the pathophysiology of gut diseases: pharmacological modulation and potential therapeutic applications. Pharmacol Ther 139:157–188
Burnstock G, Novak I (2013) Purinergic signalling and diabetes. Purinergic Signal 9:307–324
Höpker VH, Saffrey MJ, Burnstock G (1996) Neurite outgrowth of striatal neurons in vitro: involvement of purines in the growth-promoting effect of myenteric plexus explants. Int J Dev Neurosci 14:439–451
Sand E, Voss U, Hammar O et al (2012) Gonadotropin-releasing hormone analog buserelin causes neuronal loss in rat gastrointestinal tract. Cell Tissue Res 351(3):521–534
Ekblad E, Ekman R, Håkansson R, Sundler F (1988) Projections of peptide-containing neurons in rat colon. Neuroscience 27:655–674
Ekblad E, Alm P, Sundler F (1994) Distribution, origin and projections of nitric-oxide synthase-containing neurons in gut and pancreas. Neuroscience 63:233–248
Acknowledgments
We thank Anna Themner-Persson for excellent technical assistance.
Funding
This study was supported by Påhlsson foundation, Royal Physiographic Society and Faculty of Medicine, Lund University.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voss, U., Turesson, M.F., Robaye, B. et al. The enteric nervous system of P2Y13 receptor null mice is resistant against high-fat-diet- and palmitic-acid-induced neuronal loss. Purinergic Signalling 10, 455–464 (2014). https://doi.org/10.1007/s11302-014-9408-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-014-9408-5