Abstract
Mountain forest soils contain an important stock of carbon. Their altitudinal gradient can serve as a model for research on the potential risk of increased emission of carbon dioxide to the atmosphere, in a positive feedback of global warming. Using soil samples collected at three elevations (600, 900, and 1200 m a.s.l.) from five separate slopes of the Carpathian Mountains (Poland), we studied the effects of soil physical, chemical and microbial properties controlling the temperature sensitivity (Q10 values) of organic matter decomposition in forest soils. Data of soil basal respiration rate measured in laboratory conditions at six different temperatures (5, 10, 15, 20, 25 and 30 °C) were fitted to a Gaussian function. The modelled soil respiration rates differed between altitudes at temperature exceeding 15 °C, and the respiration rate of soil from 1200 m a.s.l. was higher than in soils from the two lower elevations. Based on the modelled respiration values, we calculated Q10 values in the low (Q10L, 0–10 °C), medium (Q10M, 10–20 °C) and high (Q10H, 20–30 °C) temperature ranges. The Q10 values did not differ between elevations. Q10L and Q10M were negatively related only with the C:N ratio. Temperature sensitivity of decomposition of soil organic matter was not affected by bacterial activity and functional diversity (assessed using Biolog® ECO plates), microbial biomass or community structure (inferred from phospholipid fatty acid assays). Our findings support a kinetics-based theory of the higher temperature sensitivity of more chemically recalcitrant soil organic matter, put forward by other authors.
Similar content being viewed by others
Introduction
Soil organic matter (SOM) decomposition processes are temperature dependent (Kirschbaum 2000). It has been suggested that the sensitivity of decomposition to global warming may be latitude-dependent (grater at higher than at lower latitude), leading to further acceleration of climate warming (Cox et al. 2000; Kirschbaum 2006). Mountain areas, with their vertical gradients of temperature and precipitation and lower variability of soil physicochemical properties, provide a platform for observing climate changes similar to those observed across latitudes, and can serve as a model for studying the temperature sensitivity of SOM along climatic (altitudinal) gradient.
Despite a number of studies on this important problem, it is still not clear which factors are the main drivers of SOM temperature sensitivity. Vanhala et al. (2007) found that old recalcitrant organic matter is more temperature-sensitive than young organic matter. Analysing the same soil by a slightly different method, Conen et al. (2008) reported no difference in temperature sensitivity between old and young SOM fractions. In our previous study of soils from an altitudinal climatic gradient in the Western Carpathians (Klimek and Niklińska 2010), we showed that temperature sensitivity of decomposition of SOM was negatively related to the soil C:N ratio, but the relationship was significant only under a level of soil moisture optimal for soil microorganisms activity. More recently, Schmidt et al. (2014) showed that the temperature sensitivity of SOM also depends on soil biology, that is, the microbial communities. An examination of the sensitivity of the soil organic matter to changes in temperature changes should also take into account soil microbial parameters such as functional diversity or community structure.
According to van’t Hoff’ rule, the rate of chemical processes, including metabolisms of soil microorganisms, increases two to three times with each 10 °C increase of temperature (Fang and Moncrieff 2001; Hamdi et al. 2013). However, the process rate increase is known to be higher at lower temperature ranges (Kirschbaum 1995; Niklińska et al. 1999; Tjoelker et al. 2001; Vanhala et al. 2008). It is difficult to determine exact values of this parameter, labelled Q10 due to differences between soils and ecosystems, and also between the mathematical models used (Bosatta and Ågren 1999; Fang and Moncrieff 2001; Tuomi et al. 2008; Karhu et al. 2010; Schipper et al. 2014). As shown by Tuomi et al. (2008), and later by Lellei-Kovács et al. (2011), a Gaussian model is the best one to use for predicting biochemical reactions to temperature, because it includes the decrease of the soil respiration rate after it reaches maximum at optimal temperature.
In our previous work (Niklińska and Klimek 2007; Klimek and Niklińska 2010) we showed that soils collected from different altitudes of a mountain altitudinal gradient did not differ in Q10, against our expectation of higher temperature sensitivity in soil samples from the highest altitude studied. In those previous studies we determined Q10 values based on simple calculations from respiration rates at three different temperatures. We used similar methodology in these same laboratory and thus we consider the results can be compared without bias. Here we use a Gaussian model to compare the temperature sensitivity of SOM decomposition in samples from different altitudes (measured as soil respiration rate in a range of temperatures), and we analyse the relation of Q10 with soil physicochemical parameters and with microbial community characteristics such as microbial biomass, functional diversity and community structure.
Materials and methods
Research area and soil sampling
This work uses the data published by Klimek et al. (2015) and therefore only a brief summary of some methods is given here. The data published earlier include studied soils physicochemical properties and results of Biolog® ECO plates analysis. Soil samples were collected in the Beskidy Mountains, which is a part of the Western Carpathians (southern Poland). The samples were taken in forest stands on the northern slopes of five mountain massifs built of the same bedrock, known as Carpathian flysch (multi-tiered sedimentary rocks). The research sites are at approximately the same latitude, so the temperature changes associated with the latitudinal gradient are negligible; we treated the five mountains as experimental replicates (Fig. 1).
Sampling plots (300 m2 each) were established at three elevations (600, 900, 1200 m a.s.l.) on each mountain, giving a total 15 research sites. Mean annual temperature at those elevations is as follows: 7.2 °C at 600 m a.s.l., 5.3 °C at 900 m a.s.l. and 3.8 °C at 1200 m a.s.l.. This makes a 3.4 °C decrease of mean annual temperature from the lowest to the highest stands (Klimek and Niklińska 2010). The vegetation diversity of the forest stands decreases with elevation and it changes in composition from mixed foothill forest at 600 m a.s.l. to spruce-dominated forest at 1200 m a.s.l. (Klimek et al. 2015).
At each plot, after removal of the current year’s litter we collected a sample of the upper soil horizon to 10 cm depth with a steel core sampler at three points on a horizontal transect across the slope. This procedure yielded 45 separate soil samples. The data for the three soil samples per site were averaged to avoid pseudoreplication. The samples were sieved through 1-cm mesh, followed by 0.2-cm mesh, and then stored at 4 °C.
Soil physical and chemical analyses
The soil physicochemical analyses are described in detail elsewhere (Klimek et al. 2015); they included soil dry weight (DW), soil organic matter (SOM), water holding capacity (WHC), and soil pH in water and in 1 M KCl solution. Total element concentrations (Ca, K, Mg, Mn, Na and P) in each soil sample were determined using atomic absorption spectrometry or a flow injection analyser (for P content), and accuracy was verified by running five blanks and five replicates of standard certified material (CRM025-050, Sandy Loam 8, RT Corp.). Total C, N and S were analysed using a CHNS analyser. The concentrations of dissolved organic carbon (DOC) and nitrogen (DON) were also measured.
Biolog® ECO plate analyses
The catabolic activity and functional diversity of the soil bacteria were analysed as described by Klimek et al. (2015). Briefly, we used Biolog® ECO plates containing three sets of 31 carbon substrates, with tetrazolium dye as the substrate utilisation indicator (http://www.biolog.com). Soil (equivalent of 5 g DW) was extracted and diluted (10−2) with 0.9 % NaCl solution (pH 7), and the obtained suspension was inoculated on the plates (125 µl per well). All plates were incubated in the dark at 22 °C, and substrate utilisation was measured as absorbance at 590 nm (µQuant spectrometer; BIO-TEK Instruments) at 12 h intervals for 168 h. General bacterial catabolic activity was defined as sum of areas under curve for all substrates (AUC), and the functional diversity index, H′bact, is based on the structure of substrate use (Klimek et al. 2015).
PLFA analysis
Soil microbial biomass and community structure were analysed based on phospholipid fatty acids (PLFA), following Frostegård et al. (1991). Total soil lipids were extracted from 0.5 g DW fresh soil using a one-phase mixture, the extracts were divided into two phases, the lower lipid-containing phase was collected, the lipid material was fractionated on columns containing silica acid, the phospholipid-containing polar lipids were collected, and a preparation of their fatty acid methyl esters was made by mild alkaline methanolysis. Methyl nonadecanoate (19:0) was the internal standard. The fatty acid methyl esters were separated using gas chromatography (Perkin Elmer, Claus 600 MS with FID detector) and quantified using qualitative fatty acid methyl esters mixes (Sigma–Aldrich).
The abundance of 22 extracted individual PLFAs was expressed as nanomoles of PLFA per DW gram soil, and their sum for each sample (PLFAtot) was taken as the indicator of soil microbial biomass. Fatty acid nomenclature follows Frostegård et al. (1993). The classification of individual fatty acids to bacterial and fungal biomass (PLFAbact and PLFAfungi, respectively) according to previous studies was performed according to classical studies (Frostegård et al. 1993; Hill et al. 2000; Waldrop et al. 2000). The relative shares of PLFAbact and PLFAfungi in PLFAtot were also calculated.
Respiration rate measurements and Q10 determination
The soil samples (equivalents of 2 g DW) were placed in 50-ml glass bottles, adjusted to 50 % of their maximum WHC and acclimated at 5, 10, 15, 20, 25 and 30 °C (±0.5 °C) for 5 days before respiration rate measurements. Three sets of soil samples were prepared: the first set for respiration rate measurement at 5 and 10 °C, the second for measurement at 15 and 20 °C, and the third for measurement at 25 and 30 °C. These was done in order to subject the soil samples to increased temperature as briefly as possible, to prevent depletion of the available respiratory substrate (Liu et al. 2006). Carbon dioxide evolution was measured with a 30-channel MicroOxymax respirometer (Columbus Instruments, Ohio, USA). Due to the limited number of measurement channels in the respirometer, the respiration rate was measured in series with randomly selected samples. The measurements were made in darkness for 12 h to achieve stable CO2 emission. The respiration rate of each sample was expressed as nM CO2 per g of soil organic matter per h.
To determine the temperature sensitivity of the soil respiration rate, we fitted parameter R0, a and b coefficients of the Gaussian model described by Tuomi et al. (2008):
where R0 is the respiration rate (nM CO2 g SOM−1 h−1) at 0 °C and T is temperature Celsius. The temperature-dependent Q10 curves were calculated as:
based on the fitted parameters of the R(T) functions. Q10 values were evaluated for three temperature ranges: Q10L for 0–10 °C, representing the range closest to the average annual temperature of the study area; Q10M for 10–20 °C, representing the average summer temperature; and Q10H for 20–30 °C, corresponding to summer heat waves in the studied area.
Statistical analyses
Differences between altitudes in mean soil physicochemical properties and microbial characteristics (AUC, H′bact, PLFAtot and community structure indices, used in further statistical analyses) were compared by one-way ANOVA. One-way ANOVA also was used to test differences in parameters of the R(T) function (R0, a and b coefficients), respiration rates at given temperature (°C) and Q10 values for three temperature ranges (Q10L, Q10M and Q10H). Right- or left-skewed data were transformed to fulfil the normality criterion. The Shapiro–Wilk test was used to confirm the normality of the data distribution within groups. The significance of differences between means was checked by Tukey’s test. Differences were considered significant at P < 0.05.
Multiple regression analysis was used to assess the effects of soil properties on the parameters of the R(T) function (R0, a and b), respiration rate at 16 °C (R16) and at 30 °C (R30) and Q10 values separately. In this analysis, the R0, a, b and Q10 values were used as dependent variables. Based on multiple-variable analysis, K and DOC and the C:N ratio were independent variables representing soil nutrient status for microorganisms; H′bact, bacterial biomass PLFAbact, and bacterial fraction in total microbial biomass were independent variables representing community characteristics. The percentage of total variance explained by the model is reported as the R2 value adjusted for degrees of freedom \(\left( {{\text{R}}^{ 2}_{\text{adj}} } \right)\). Statistical analyses were employed Statgraphics Centurion XVI software (StatPoint Technologies Inc., Warrenton, VA, USA).
Results
Physicochemical and microbiological properties of studied soils
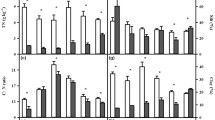
Soil samples from the three altitudes differed significantly only in mean of SOM content, water holding capacity (WHC) and dissolved organic nitrogen (DON) content (Table 1). The soils from sites at the highest elevation (1200 m a.s.l.) showed the highest organic matter content (41.2 %) and higher WHC (446 %) than those at the lower two elevations. The DON concentration was highest in soils at 1200 m a.s.l. (0.048 mg g−1 DW), differing significantly from soils at 600 m a.s.l., which had the lowest DON value (0.021 mg g−1 DW). Potassium content and soil C:N ratio did not differ between elevations (Table 1). The values for other physicochemical parameters are given in Klimek et al. (2015).
Mean H′bact values differed between altitudes, ranging from 0.87 in soil under spruce forest at 1200 m a.sl. to 1.06 in soil under mixed forest in foothills at 600 m a.s.l. (Table 1). In contrast, AUC, PLFAtot and microbial community structure indices calculated from PLFA data did not differed between elevations (Table 1).
Multiple-variable analysis showed high correlations between many of the measured soil physicochemical and microbiological properties (Table S1). For example, the soil C:N ratio was positively correlated with the soil C:P and C:S ratios, PLFAtot and PLFAfungi and negatively correlated with soil pH. In turn, DOC was correlated positively with DON but negatively with Mg and Mn content (Table S1).
Soil respiration rate, Q10 and their relation to soil factors
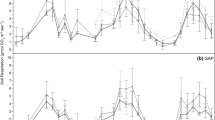
The Gaussian model of the temperature dependence of soil respiration fit the measured data well, and the models fitting adjusted for degrees of freedom \(\left( {R^{ 2}_{\text{adj}} } \right)\) explained from 97.0 to 99.6 % variability in given temperatures (data not shown). R0 representing the respiration rate at 0 °C, serving as a proxy of SOM decomposability, did not differ between elevations (P = 0.1067) (Table 1). At higher temperatures (≥16 °C) there were differences in mean modelled respiration rate between soils from different altitudes (Table 1). In the 16–30 °C range the modelled soil respiration rate was significantly higher for soils from 1200 m a.s.l. than for soils from lower altitudes (Fig. 2). The respiration rate of soils from 1200 m a.s.l. was about three times higher than that of soils from lower altitudes (Table 1).
Relation of a soil respiration rate and b soil temperature sensitivity to temperature in soils from 600 (filled squares), 900 (filled triangles) and 1200 m a.s.l. (filled dots). Gaussian functions describe temperature dependence of soil respiration. The Q10 curves were calculated using R0, a and b parameters of Gaussian function averaged (n = 5) for each of the studied elevations
Coefficients a and b of the Gaussian model did not differed between elevations (Table 1). For soil from all elevations, the temperature-dependent Q10 values calculated based on the respiration rate values fitted to Gaussian functions showed decreasing temperature sensitivity with the increase of test temperature (Fig. 2). The differences in Q10 between altitudes were not significant in any of the tested temperature ranges (Table 1).
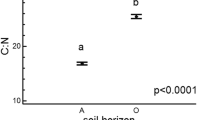
Multiple regression yielded significant models for R0, R16, and R30 and for Q10L and Q10M values (P values ranging from 0.0384 to less than 0.0001). The models explained from 25.5 % (Q10L) to 87.4 % (R0) of the variance of these values (Table 2). R0 was correlated positively to the DOC content and the soil C:N ratio (as well as R16 and R30 were; data not shown). Q10L and Q10M were correlated only (and negatively) with the soil C:N ratio (Table 2).
Discussion
The soils from the studied altitudes differed significantly in only some of the measured physicochemical and microbiological properties. Although the respiration rate found for soil from the highest studied altitude (1200 m a.s.l.) was higher than for soil taken from lower altitudes, we did not find differences in temperature sensitivity of decomposition of SOM between elevations; the study did not confirm higher temperature sensitivity of soils from colder sites than from warmer sites. This result is in line with findings from our earlier study of an altitudinal gradient in the Western Carpathians (Niklińska and Klimek 2007; Klimek and Niklińska 2010).
In a current study, the calculated Q10 values were temperature-dependent, decreasing with the increase of test temperature. In the 0–10 °C range the Q10 values were 2.8–3.2, exceeding the 2.5 value reported as a mean for forest ecosystems (Hamdi et al. 2013) and the 2.0–2.5 range often used in global C cycling models (Ise and Moorcroft 2006). Although the difference in Q10 is not large and the soil respiration rate at lower temperature is generally low, such temperature sensitivity may herald a pronounced increase in soil carbon loss to atmosphere (Kähkönen et al. 2001), because such conditions are typical for the large earth areas during the greater part of the vegetation season.
We expected that temperature sensitivity of soil organic matter decomposition processes along mountain altitudinal gradient would differ between altitudes. The lack of differences in Q10 between altitudes, as we found, may be due to too short studied gradient but also differences between stands from the same elevation. On the other hand, the lack of these differences may resulted from stands unifying, resulting from human activity (e.g. selective tree cutting or spruce introduction below natural belt) that altered the soil processes of some stands. Such anthropopression may be reflected in the lack of large differences in soil physicochemical and microbiological characteristics between altitudes.
Multiple regression indicated that the soil respiration rate modelled with a Gaussian function was positively correlated with the soil C:N ratio and DOC concentration. This demonstrates the importance of readily degradable carbon compounds for soil microorganisms metabolic activity. Demoling et al. (2007) found that soil bacteria can be limited by carbon availability even in soils with a wide C:N ratio. In our current study on soils from mountain altitudinal gradient, the soil C:N ratio was positively correlated with soil microbial biomass, including fungal biomass as measured by the PLFA method. High microbial biomass is related to high microbial activity, but carbon availability may affect microbial activity and biomass differently (Allen and Schlesinger 2004; Spohn and Chodak 2015). The soils from the highest altitude studied, having higher SOM content, showed a higher respiration rate modelled with a Gaussian function for temperatures above 16 °C. However, the soils from the different altitudes did not differ significantly in C:N ratio, DOC content or microbial biomass. These results suggest site-specific differences unrelated to their substrate (the sites are all on the same bedrock, Carpathian flysch).
Multiple regression analysis indicated that Q10 in the low and medium temperature range was correlated negatively with the soil C:N ratio. Such a trend, opposite to the results for respiration rate, seems to support the kinetic-based suggestion of higher temperature sensitivity of more chemically recalcitrant SOM and its associated soil microbial communities (Vanhala et al. 2007). That idea was reconsidered recently by Xu et al. (2015), who made a long-term laboratory study of the temperature sensitivity of soils along an altitudinal gradient in the Wuyi Mountains (China). They showed that soils with higher soil organic carbon recalcitrance showed significantly higher temperature sensitivity of decomposition of SOM. Their results also indicate that the temperature sensitivity of both the labile and the recalcitrant SOM fractions is negatively correlated with the soil C:N ratio in stands delineated at these same altitudes. Similar results were given by Hilasvuori et al. (2013) in their study of temperature sensitivity along the peat profile; the oxic peat layer, with the lowest C:N ratio, showed the highest temperature sensitivity. A low soil C:N ratio may, however, have two different explanations driven by completely different ecological processes. One explanation is that a high rate of SOM decomposition causes depletion of thee carbon and a relative increase of nitrogen; this can take place in conditions favourable for fast decomposition. A low soil C:N ratio may also result from the chemical composition of litter, and may depend more on the quality of litter produced in a given ecosystem.
Against expectation, we did not find that the Q10 coefficients were related to bacterial functional diversity. Apparently, more functionally diverse communities are able to effectively degrade various organic substrates and, as a result, can take more advantage of increasing temperature. The lack of such relationships may result from carbon availability restrictions influencing bacterial performance, or may be due to limitations of the method, that is, the Biolog® plates (Campbell et al. 2003). Vanhala et al. (2008) also found that the results from Biolog® plates did not correlate with Q10 based on a Gaussian function. Our findings may also suggest a stronger effect on temperature sensitivity of decomposition of soil organic matter from the fungal part of community than from bacteria. There is in line with findings of Pietikäinen et al. (2005), who found that reaction of growth rate to temperature increase was more drastic for fungi than for bacteria. That possibility needs to be studied further.
Conclusions
The Q10 parameters describing temperature sensitivity of SOM were temperature-dependent and decreased with the increase of test temperature. In the lower temperature range, typical for the study area, we found the Q10 values of around 3, higher than the values typically used in climate models. We did not find differences in Q10 between altitudes differing in mean annual temperature, which suggest a lack of higher temperature sensitivity in soil of colder regions or may result from too short climatic gradient studied. The Q10 values in the low and medium temperature ranges (0–10 °C, 10–20 °C) were negatively correlated with the C:N ratio. These findings are in line with the kinetics-based suggestion of the higher temperature sensitivity of more chemically recalcitrant SOM and its associated soil microbial communities.
References
Allen AS, Schlesinger WH (2004) Nutrient limitations to soil microbial biomass and activity in loblolly pine forests. Soil Biol Biochem 36:581–589
Bosatta E, Ågren GI (1999) Soil organic matter quality interpreted thermodynamically. Soil Biol Biochem 31:1889–1891
Campbell CD, Chapman SJ, Cameron CM, Davidson M, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol 69:3593–3599
Conen F, Karhu K, Leifeld J, Seth B, Vanhala P, Liski J, Alewell C (2008) Temperature sensitivity of young and old soil carbon—same soil, slight differences in 13C natural abundance method, inconsistent results. Soil Biol Biochem 40:2703–2705
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187
Demoling F, Figueroa D, Bååth E (2007) Comparison of factors limiting bacterial growth in different soils. Soil Biol Biochem 39:2485–2495
Fang C, Moncrieff JB (2001) The dependence of soil CO2 efflux on temperature. Soil Biol Biochem 33:155–165
Frostegård A, Tunlid A, Bååth E (1991) Microbial biomass measured as total lipid phosphate in soils of different organic content. J Mic Meth 14:151–163
Frostegård Å, Tunlid A, Bååth E (1993) Phospholipid fatty acid composition, biomass, activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Mic 59:3605–3617
Hamdi S, Moyano F, Sall S, Bernoux M, Chevallier T (2013) Synthesis analysis of the temperature sensitivity of soil respiration from laboratory studies in relation to incubation methods and soil conditions. Soil Biol Biochem 58:115–126
Hilasvuori E, Akujärvi A, Fritze H, Karhu K, Laiho R, Mäkiranta P, Oinonen M, Palonen V, Vanhala P, Liski J (2013) Temperature sensitivity of decomposition in a peat profile. Soil Biol Biochem 67:47–54
Hill GT, Mitkowski NA, Aldrich-Wolfe L, Emele LR, Jurkonie DD, Ficke A, Maldonado-Ramirez S, Lynch ST, Nelson EB (2000) Methods for assessing the composition and diversity of soil microbial communities. Appl Soil Ecol 15:25–36
Ise T, Moorcroft PR (2006) The global-scale temperature and moisture dependencies of soil organic carbon decomposition: an analysis using a mechanistic decomposition model. Biogeochemistry 80:217–231
Kähkönen A, Wittman C, Kurol J, Ilvesniem H, Salkinoja-Salone MS (2001) Microbial activity of boreal forest soil in a cold climate. Boreal Environ Res 6:19–28
Karhu K, Fritze H, Tuomi M, Vanhala P, Spetz P, Kitunen V, Liski J (2010) Temperature sensitivity of organic matter decomposition in tow boreal forest soil profiles. Soil Biol Biochem 42:72–82
Kirschbaum MUF (1995) The temperature dependence of soil organic matter decomposition and the effect of global warming on soil organic carbon storage. Soil Biol Biochem 27:753–760
Kirschbaum MUF (2000) Will changes in soil organic matter act as a positive or negative feedback on global warming? Biogeochemistry 48:21–51
Kirschbaum MUF (2006) The temperature dependence of organic-matter decomposition—still a topic of debate. Soil Biol Biochem 38:2510–2518
Klimek B, Niklińska M (2010) Decomposition of organic matter from two soil layers along an altitudinal climatic gradient is differently affected by temperature and moisture. Pol J Environ Studies 19:1229–1237
Klimek B, Niklińska M, Jaźwa M, Tarasek A, Tekielak I, Musielok Ł (2015) Covariation of soil bacteria functional diversity and vegetation diversity along an altitudinal climatic gradient in the Western Carpathians. Pedobiologia 58:105–112
Lellei-Kovács E, Kovács-Láng E, Botta-Dukát Z, Kalapos T, Emmett B, Beier C (2011) Thresholds and interactive effects of soil moisture on the temperature response of soil respiration. Eur J Soil Biol 47:247–255
Niklińska M, Klimek B (2007) Effect of temperature on the respiration rate of forest soil organic layer along an elevation gradient in the Polish Carpathians. Biol Fertil Soils 43:511–518
Niklińska M, Maryanski M, Laskowski R (1999) Effect of temperature on humus respiration rate and nitrogen mineralization: implications for global climate change. Biogeochemistry 44:239–257
Pietikäinen J, Pettersson M, Bååth E (2005) Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiol Ecol 52:49–58
Schipper LA, Hobbs JK, Rutledge S, Arcus VL (2014) Thermodynamic theory explains the temperature optima of soil microbial processes and high Q10 values at low temperatures. Global Change Biol 20:3578–3586
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2014) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Spohn M, Chodak M (2015) Microbial respiration per unit biomass increases with carbon-to-nutrient ratios in forest soil. Soil Biol Biochem 81:128–133
Tjoelker MG, Oleksyn J, Reich PB (2001) Modelling respiration of vegetation: evidence for a general temperature-dependent Q10. Global Change Biol 7:223–230
Tuomi M, Vanhala P, Karhu K, Fritze H, Liski J (2008) Heterotrophic soil respiration—comparison of different models describing its temperature dependence. Ecol Modell 211:182–190
Vanhala P, Karhu K, Tuomi M, Sonninen E, Jungner H, Fritze H, Liski J (2007) Old soil carbon is more temperature sensitive than the young in an agricultural field. Soil Biol Biochem 39:2967–2970
Vanhala P, Karhu K, Tuomi M, Björklöf K, Fritze H, Liski J (2008) Temperature sensitivity of soil organic matter decomposition in southern and northern areas of the boreal forest zone. Soil Biol Biochem 40:1758–1764
Xu Z, Tang S, Xiong Li, Yang W, Yin H, Tu L, Wu F, Chen L, Tan B (2015) Temperature sensitivity of soil respiration in China’s forest ecosystems: patterns and controls. Appl Soil Ecol 93:105–110
Waldrop M, Balser T, Firestone M (2000) Linking microbial community composition to function in a tropical soil. Soil Biol Biochem 32:1837–1846
Acknowledgments
Michael Jacobs line-edited the paper for submission. This study was supported by a grant from the Polish State Committee for Scientific Research (0594/B/P01/2011/40) and by Jagiellonian University funds (subsidy DS/WBiNoZ/INoS/758).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Klimek, B., Jelonkiewicz, Ł. & Niklińska, M. Drivers of temperature sensitivity of decomposition of soil organic matter along a mountain altitudinal gradient in the Western Carpathians. Ecol Res 31, 609–615 (2016). https://doi.org/10.1007/s11284-016-1369-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-016-1369-4