Abstract
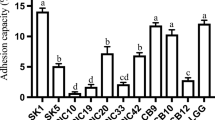
The aim of this study was to investigate some probiotic properties of 42 wild Lactobacillus plantarum strains isolated from different Italian foods of animal origin. The strains were first screened for their antibiotic resistance profile (chloramphenicol, erythromycin, gentamicin, and tetracycline), subsequently they were tested for their in vitro resistance to lysozyme (100 mg L−1), low pH (3.0, 2.5 and 2.0) and bile salts (0.3, 0.5 and 1.0 %). Moreover, agglutination property was studied (adhesion to Saccharomyces cerevisiae cells), as well as the presence of bsh and msa genes. The strains with the best characteristics were subjected to a further trial in order to evaluate their ability to survive to multiple stresses over time (lysozyme, low pH and bile salts) and the effect of these treatments on adhesion to yeast cells. All the strains were susceptible to chloramphenicol, erythromycin and gentamicin, while 6 strains were excluded from further evaluation because of their resistant phenotype against tetracycline. All the strains were able to grow in presence of lysozyme, as well as in MRS broth at pH 3.0. Only 4 strains showed a growth rate lower than 80 % when grown in MRS broth at pH 2.5, while a relevant growth rate decrease was observed after exposure to pH 2.0. Bile salts didn’t affect the viability of the L. plantarum cells. Twenty-one strains out of 33 tested strains were able to adhere to S. cerevisiae cells. Presence of both bsh and msa genes was detected in 6 strains. The strains resistant to all the stresses, positive to agglutination with S. cerevisiae and showing bsh and msa genes were selected for further evaluation and subjected to different stress treatments over time. The assessment of growth rates showed that exposure to lysozyme significantly increased low pH resistance in L. plantarum. This increase ranged from 2.35 to 15.57 %. The consequential lysozyme and low pH exposures didn’t affect the growth rate values after bile salts treatment, as well as the ability of the strains to adhere to yeast cells wasn’t modified by previous treatments (lysozyme, low pH and bile salts). The present work allows to increase knowledge about non starter lactic acid bacteria from Italian food products. The studied L. plantarum strains showed a good potential for their use as probiotic cultures. However, more in vivo tests are necessary to confirm this potentiality.
Similar content being viewed by others
References
Berthier F, Ehrlich SD (1998) Rapid species identification within two groups of closely related lactobacilli using PCR primers that target the 16S/23S rRNA spacer region. FEMS Microbiol Lett 161:97–106
Bosch M, Rodriguez M, Garcia F, Fernàndez E, Fuentes MC, Cuné J (2011) Probiotic properties of Lactobacillus plantarum CECT 7315 and CECT 7316 isolated from faeces of healthy children. Lett Appl Microbiol 54:240–246
Charteris WP, Kelly PM, Morelli L, Collins JK (1998) Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J of Appl Microbiol 84:759–768
Collado MC, Surono I, Meriluoto J, Salminen S (2007) Indigenous dadih lactic acid bacteria: cell-surface properties and interactions with pathogens. J Food Sci 72:89–93
Cunningham FE, Proctor VA, Goetsch SJ (1991) Egg-white lysozyme as a food preservative: an overview. World Pollut Sci J 47:141–163
De Angelis M, Corsetti A, Tosti N, Rossi J, Corbo MR, Gobbetti M (2001) Characterization of Non-Starter lactic acid bacteria from Italian ewe cheeses based on phenotypic, genotypic, and cell wall protein analyses. Appl Environ Microbiol 67:2011–2020
De Vries MC, Vaughan EE, Kleerebezem M, de Vos WM (2006) Lactobacillus plantarum: survival, functional and potential probiotic properties in the human gastrointestinal tract. Int Dairy J 16:1018–1028
Del Re B, Sgorbati B, Miglioli M, Palenzona D (2000) Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 31:438–442
EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (2012) Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10(6):2740 [10 pp.] doi:10.2903/j.efsa.2012.2740
Egervärn M, Roos S, Lindmark H (2009) Identification and characterization of antibiotic resistance genes in Lactobacillus reuteri and Lactobacillus plantarum. J Appl Microbiol 107:1658–1668
FAO/WHO (2001) Report on joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria
Georgieva R, Iliev I, Haertlé T, Chobert JM, Ivanova I, Danova S (2009) Technological properties of candidate probiotic Lactobacillus plantarum strains. Int Dairy J 19:696–702
Gevers D, Huys G, Swings J (2003) In vitro conjugal transfer of tetracycline resistance from Lactobacillus isolates to other Gram-positive bacteria. FEMS Microbiol Lett 225:125–130
Gross G, Snel J, Boekhorst J, Smits MA, Kleerebezem M (2010) Biodiversity of mannose-specific adhesion in Lactobacillus plantarum revisited: strain-specific domain composition of the mannose-adhesin. Beneficial Microbes 1(1):61–66
Heneghan JB (1988) Alimentary tract physiology: interaction between the host and its microbial flora. In: Rowland IR (ed) Role of the Gut Flora in Toxicity and Cancer. Academic Press, San Diego, CA, pp 39–78
Hentges DJ (1992) Gut flora and disease resistance. In: Fuller R (ed) (Hrsg): probiotics. Chapman and Hall, London, pp 87–110
Holzapfel WH, Haberer P, Snel J, Björkroth J, Schillinger U, Huis in’t Veld JHJ (1998) Overview of gut flora and probiotics. Int J Food Microbiol 41:85–100
Jensen H, Grimmer S, Naterstad K, Axelsson L (2012) In vitro testing of commercial and potential probiotic lactic acid bacteria. Int J Food Microbiol 153:216–222
Kaushik JK, Kumar A, Duary RK, Mohanty AK, Grover S, Batich VK (2009) Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS One. doi:10.1371/journal.pone.0008099
Klare I, Konstabel C, Müller-Bertling S, Reissbrodt R, Huys G, Vancanneyt M, Swings J, Goossens H, Witte W (2005) Evaluation of new broth media for microdiluition antibiotic susceptibility testing of lactobacilli, pediococci, lactococci and bifidobacteria. Appl Environ Microbiol 71:8982–8986
Klayraung S, Viernstein H, Sirithunyalug J, Okonogi S (2008) Probiotic properties of lactobacilli isolated from Thai traditional food. Sci Pharm 76:485–503
Luckey TD, Floch MH (1972) Introduction to intestinal microbiology. Am J Clin Nutr 25:1291–1295
Mathara JM, Schillinger U, Kutima PM, Mbugua SK, Guigas C, Franz C, Holzapfel WH (2008) Functional properties of Lactobacillus plantarum strains isolated from Maasai traditional fermented milk products in Kenya. Curr Microbiol 56:315–321
Nagata Y, Hashiguchi K, Kamimura Y, Yoshida M, Gomyo T (2009) The gastointestinal transit tolerance of Lactobacillus plantarum strain no. 14 depended on the carbon source. Biosci Biotechnol Biochem 73:2650–2655
Patel AK, Singhania RR, Pandey A, Chincholkar SB (2010) Probiotic bile salt hydrolase: current developments and perspectives. Appl Biochem Biotechnol 162:166–180
Pisano MB, Patrignani F, Cosentino S, Guerzoni ME, Franz CMAP, Holzapfel WH (2010) Diversity and functional properties of Lactobacillus plantarum-group strains isolated from Italian cheese products. Dairy Sci Technol. doi:10.1051/dst/2010037
Pretzer G, Snel J, Molenaar D, Wiersma A, Bron PA, Lambert J, de Vos WM, van der Meer R, Smits MA, Kleerebezem M (2005) Biodiversity-based identification and functional characterization of the mannose-specific adhesion of Lactobacillus plantarum. J Bacteriol 17:6128–6136
Rodgers S (2008) Novel applications of live bacteria in food services: probiotics and protective cultures. Trends Food Sci Tech 19:188–197
Vinderola CG, Reinheimer JA (2003) Lactic acid starter and probiotic bacteria: a comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Research Int 36:895–904
Vizoso Pinto MG, Franz CMAP, Schillinger U, Holzapfel WH (2006) Lactobacillus spp. with in vitro probiotic properties from human faeces and traditional fermented products. Int J Food Microbiol 109:205–214
Zago M, Fornasari ME, Carminati D, Burns P, Suarez V, Vinderola G, Reinheimer J, Giraffa G (2011) Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheese. Food Microbiol 28:1033–1040
Zhang L, Zhang X, Liu C, Li C, Li S, Li T, Li D, Zhao Y, Yang Z (2012) Manufacture of cheddar cheese using probiotic Lactobacillus plantarum K25 and its cholesterol-lowering effects in a mice model. World J Microbiol Biotechnol. doi:10.1007/s11274-012-1165-4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turchi, B., Mancini, S., Fratini, F. et al. Preliminary evaluation of probiotic potential of Lactobacillus plantarum strains isolated from Italian food products. World J Microbiol Biotechnol 29, 1913–1922 (2013). https://doi.org/10.1007/s11274-013-1356-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1356-7