Abstract
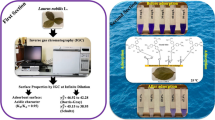
Tobacco (Nicotiana tabacum L.) stem ash (TSA) was evaluated as an adsorbent for removal of methylene blue (MB) from aqueous solution by batch adsorption method. MB adsorption increased with increase in contact time, initial solution pH, and adsorbent dose. Contact time for adsorption equilibrium was 180 min. The MB adsorption per unit mass of adsorbent (in milligram per gram) increased with the increasing initial dye concentration. Adsorption of MB onto TSA followed the pseudo-second-order kinetic model with a rate constant (k 2) of 0.017 g mg−1 min−1. The mechanism of adsorption was described with intra-particle diffusion model. It was found that the intra-particle diffusion was not a sole rate-controlling step. Equilibrium adsorption was investigated by the Freundlich, Langmuir, Temkin, and Jovanoic isotherms. On the basis of coefficient of determination, the order of isotherm fit was Langmuir (R 2 = 0.974) > Freundlich (R 2 = 0.957) = Temkin (R 2 = 0.957) > Jovanoic (R 2 = 0.764) isotherm. The maximum monolayer adsorption capacity of TSA was 35.7 mg g−1. The dimensionless separation factor (R L) was low (0.137), indicating favorable adsorption of MB onto TSA. The results clearly demonstrate the potential of TSA as a low-cost and an easily available adsorbent for sequestering MB from wastewater.









Similar content being viewed by others
References
Abd EI-Latif, M. M., Ibrahim, A. M., & EI-Kady, M. F. (2010). Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using biopolymer oak sawdust composite. Journal of American Science, 6(6), 267–283.
Annadurai, G., Juang, R., & Lee, D. (2002). Use of cellulose based wastes for adsorption of dyes from aqueous solutions. Journal of Hazardous Materials, B92(3), 263–274.
Arias, F., & Sen, T. K. (2009). Removal of zinc metal ion (Zn2+) from its aqueous solution by kaolin clay mineral: a kinetic and equilibrium study. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 348, 100–108.
Aygun, A., Yenisoy-Karakas, S., & Duman, I. (2003). Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Material, 66(2–3), 189–195.
Bazrafshan, E., Mostafapour, F., & Zazouli, M. A. (2012). Methylene blue (cationic dye) adsorption into Salvadora persica stems ash. African Journal of Biotechnology, 11(101), 16661–16668.
Bhavornthanayod, C., & Rungrojchaipon, P. (2009). Synthesis of zeolite a membrane from rice husk ash. Journal of Metals, Materials and Minerals, 19(2), 79–83.
Chen, X. G., Lv, S. S., Liu, S. T., Zhang, P. P., Zhang, A. B., Sun, J., et al. (2012). Adsorption of methylene blue by rice hull ash. Separation Science and Technology, 47(1), 147–156.
Dawood, S., & Sen, T. K. (2012). Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Research, 46(6), 1933–1946.
Feng, Y., Zhou, H., Liu, G., Qiao, J., Wanga, J., Lu, H., et al. (2012). Methylene blue adsorption onto swede rape straw (Brassica napus L.) modified by tartaric acid: Equilibrium, kinetic and adsorption mechanisms. Bioresource Technology, 125, 138–144.
Fernandez, M. E., Nunell, G. V., Bonelli, P. R., & Cukierman, A. L. (2010). Effectiveness of Cupressus sempervirens cones as biosorbent for the removal of basic dyes from aqueous solutions in batch and dynamic modes. Bioresource Technology, 101(24), 9500–9507.
Ferrero, F. (2007). Dye removal by low cost adsorbents: Hazelnut shells in comparison with wood sawdust. Journal of Hazardous Materials, 142(1–2), 144–152.
Fiol, N., & Villaescusa, I. (2009). Determination of sorbent point zero charge: usefulness in sorption studies. Environmental Chemistry Letters, 7(1), 79–84.
Freundlich, H. (1906). Adsorption in solution. Physical Chemical Society, 40, 1361–1368.
Gupta, V. K., Mohan, D., Sharma, S., & Sharma, M. (2000). Removal of basic dyes (Rhodamine B and Methylene Blue) from aqueous solutions using bagasse fly ash. Separation Science and Technology, 35(13), 2097–2113.
Hameed, B. H., Mahmoud, D. K., & Ahmad, A. L. (2008). Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch waste. Journal of Hazardous Materials, 158, 65–72.
Jovanovic, D. S. (1969). Physical adsorption of gases I: Isotherms for monolayer and multilayer adsorption. Colloid & Polymer Science, 235, 1203–1214.
Kavitha, D., & Namasivayam, C. (2007). Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresource Technology, 98(1), 14–21.
Khattri, S. D., & Singh, M. K. (2000). Colour removal from synthetic dye wastewater using a bioadsorbent. Water, Air, and Soil Pollution, 120, 283–294.
Lal, R. (2005). World crop residues production and implications of its use as a biofuel. Environment International, 31(4), 575–584.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica, and platinum. Journal of the American Chemical Society, 40, 1361–1368.
Lin, J. X., Zhan, S. L., Fang, M. H., Qian, X. Q., & Yang, H. (2008). Adsorption of basic dye from aqueous solution onto fly ash. Journal of Hazardous Materials, 87(1), 193–200.
Marungrueng, K., & Pavasant, P. (2007). High performance biosorbent (Caulerpa lentillifera) for basic dye removal. Bioresource Technology, 98(8), 1567–1572.
Montano, J. G., Torrades, F., Perez Estrada, L. A., Oller, I., Malato, S., Maldonado, M. I., et al. (2008). Degradation pathways of the commercial reactive azo dye Procion Red H-E7B under solar-assisted photo-Fenton reaction. Environmental Science and Technology, 42(17), 6663–6670.
Mukhlish, M. Z. B., Khan, M. R., Bhoumick, M. C., & Paul, S. (2012). Papaya (Carica papaya L.) leaf powder: Novel adsorbent for removal of methylene blue from aqueous solution. Water, Air, and Soil Pollution, 223(8), 4949–4958.
Rafatullah, M., Sulaiman, O., Hashim, R., & Ahmad, A. (2010). Adsorption of methylene blue on low-cost adsorbents; a review. Journal of Hazardous Materials, 177, 70–80.
Salleh, M. A. M., Mahmoud, D. K., Karim, W. A., & Idris, A. (2011). Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination, 280(1–3), 1–13.
Sarkar, A., Rano, R., Udaybhanu, G., & Basu, A. K. (2006). A comprehensive characterization of fly ash from a thermal power plant in Eastern India. Fuel Processing Technology, 87, 259–277.
Sarkar, D., & Bandyopadhyay, A. (2010). Adsorptive mass transport of dye on rice husk ash. Journal of Water Resource and Protection, 2, 424–431.
Sen, T. K., Afroze, S., & Ang, H. (2011). Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiata. Water, Air, and Soil Pollution, 218(1–4), 499–515.
Sharma, P., & Kaur, H. (2011). Sugarcane bagasse for the removal of erythrosin B and methylene blue from aqueous waste. Applied Water Science, 1(3–4), 135–145.
Somasekhara Reddy, M. C., Sivaramakrishna, L., & Varada Reddy, A. (2012). The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. Journal of Hazardous Materials, 203–204, 118–127.
Temkin, M. J., & Pyzhev, V. (1940). Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS, 12, 217–222.
Tsai, W. T., Hsu, H. C., Su, T. Y., Lin, K. Y., & Lin, C. M. (2008). Removal of basic dye (methylene blue) from wastewaters utilizing beer brewery waste. Journal of Hazardous Materials, 154(1–3), 73–78.
Vadivelan, V., & Kumar, K. V. (2005). Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. Journal of Colloid and Interface Science, 286(1), 90–100.
Wang, S., & Zhu, Z. H. (2005). Sonochemical treatment of fly ash for dye removal from wastewater. Journal of Hazardous Materials, 126(1–3), 91–95.
Wang, S., Boyjoo, Y., & Choueib, A. (2005). A comparative study of dye removal using fly ash treated by different methods. Chemosphere, 60(10), 1401–1407.
Weng, C. H., & Pan, Y. F. (2006). Adsorption characteristics of methylene blue from aqueous solution by sludge ash. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 274(1), 154–162.
Yagub, M. T., Sen, T. K., & Ang, H. M. (2012). Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water, Air, and Soil Pollution, 223(8), 5267–5282.
Zhang, W., Li, H., Kan, X., Dong, L., Yan, H., Jiang, Z., et al. (2012). Adsorption of anionic dyes from aqueous solutions using chemically modified straw. Bioresource Technology, 117, 40–47.
Acknowledgments
The authors wish to thank the Director, C.T.R.I., for extending all facilities required for this research, and M. K. Rupnath, Mrs. N. Ramalakshmi, and the technical staff of CC and SS Division, C.T.R.I., for their assistance throughout the course of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, R.K., Reddy, D.D. Tobacco Stem Ash as an Adsorbent for Removal of Methylene Blue from Aqueous Solution: Equilibrium, Kinetics, and Mechanism of Adsorption. Water Air Soil Pollut 224, 1582 (2013). https://doi.org/10.1007/s11270-013-1582-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1582-5